EXTRACTION. Extraction is a very common laboratory procedure used when isolating or purifying a product.
|
|
|
- Paula Gray
- 5 years ago
- Views:
Transcription
1 EXTRACTION Extraction is a very common laboratory procedure used when isolating or purifying a product. Extraction is the drawing or pulling out of something from something else. By far the most universal and ancient form of extraction is the brewing of tea or the making of coffee. Over the centuries, humans have carried out solid/liquid extraction by brewing just about every common plant leaf, fruit, or root. In the process, they have isolated a number of extracts with pharmacological activity. Many of these compounds were used for medicinal purposes. While solid/liquid extraction is the most common technique used to brew beverages and isolate natural products, liquid/liquid extraction is a very common method used in the organic laboratory. Organic reactions often yield a number of by-products, some inorganic, some organic. Also, since they do not go to 100% completion, some starting material is also often present at the end of an organic reaction. Liquid/ liquid extraction is often used as the initial step in the work-up of a reaction, before final purification of the product by recrystallization, distillation, sublimation or chromatography. Liquid-liquid extraction requires two immiscible liquids known as the organic phase and the aqueous phase. The aqueous phase is water-based and the organic phase is an organic solvent. The basis of extractive techniques is the like dissolves like rule. Water typically dissolves inorganic salts (such as lithium chloride) and other ionized species, while solvents (ethyl acetate, methylene chloride, diethyl ether, etc.) dissolve neutral organic molecules. However, some compounds (e.g., alcohols) exhibit solubility in both media. Therefore, it is important to remember that this method of separation relies on partitioning that is, the preferential dissolution of a species into one solvent over another. Forexample,2-pentanol is some what soluble in water (i.e.,17g/100 ml H 2O), but infinitely soluble in diethylether. Thus 2-pentanol can be preferentially partitioned into ether. General extraction procedure: Place the solution to be extracted in the separatory funnel. As the organic solvent and water are not miscible with each other, you should be able to see the two layers (organic and aqueous layers) clearly. Now, shake the separatory funnel to increase the contact between these substances and the water. When finished, the funnel can be returned to the stand and the layers are allowed to separate. You should also have two beakers ready, one labelled "organic layer" and the other labelled "aqueous layer". Which solvent is to be used depends on the solubility of the substance to be extracted in the solvent and whether it is suitable for subsequent crystallization or distillation. The common solvent which is used for the extraction are diethyl ether, benzene, etc., *There are four extraction methods: 1) Solvent extraction 2) Extraction based on chemical reaction
2 3) sequential liquid liquid extractions 4) Solid extraction 1) SOLVENT EXTRACTION Extraction with solvents is used as a method of separation of dissolved substances from solutions. Extractions made from solvents are based on the "law of disintegration of Nerst". Distribution law or the Nernst's distribution law gives a generalisation which governs the distribution of a solute between two non miscible solvents. Nernst Distribution law. At constant temperature, a solute distributes itself between two immiscible solvents only in a particular ratio. The Nernst distribution law permits us to determine the most favorable conditions for the extraction of substances from solutions. *The Nernst distribution law states that, at equilibrium, the ratio of the concentrations of a third component in two liquid phases is constant. The law may be expressed in the form The Nernst distribution law permits us to determine the most favorable conditions for the extraction of substances from solutions. **The purpose in extraction is to be able to distinguish both organic matter and not to waste too much solvent. * When V ml aqueous solution containing M grams of compound is extracted with X ml organic solvent, M1 g compound remains in the aqueous phase. or The same amount is subjected to a second extraction using X ml of organic solvent, the following equations can be written for the M2 g substance remaining in the aqueous phase. or If the same process is done n times, the amount of substance remain in the water phase is Mn;
3 *A good solvent for extraction should satisfy two important conditions. (a) The substance to be extracted should be highly soluble in the solvent. (b) After the extraction the solvent should be easily separable from the solute. The mixture of urea and benzoic acid can be separated using solvent extraction process. The mixtures are taken in a separating funnel, to the substances (Urea and benzoic acid) which is solid, diethyl ether is added. The two mixtures are shaken well to get a solution. Only benzoic acid is soluble in ether, while urea is not. Urea is at the bottom of the separating funnel, and it is collected. Now the benzoic acid in ether is left, which on heating, we get benzoic acid. *During extraction process undesirable conditions may be encountered, such as non-separation of phases or emulsion formation. In this case: -The aqueous phase may be saturated with sodium chloride. -If the solvent is suitable, a few drops of alcohol may be added. - It may be waited for a while. -Air may be blown through the water phase with a pipette. -It may be heated. 2) EXTRACTİON BASED ON CHEMİCAL REACTİON In this type of extraction, the substance to be separated gives a chemical reaction with the extraction solution. For example, an organic acid which dissolved in an organic solvent with a few other substances, can be pass into the aqueous phase by creating a salt form after an organic reaction with an aqueous solution of an inorganic base. 3) SEQUENTIAL LIQUID LIQUID EXTRACTIONS This continuous extraction is carried out when organic substances dissolve more in water than in organic solvents or in a solid phase and are less soluble in organic solvents. In this method, even though the solubility is against the organic solvent, more substances can be recovered by using less solvent. 4) EXTRACTION FROM SOLID DRUGS Extraction, as the term is used pharmaceutically, involves also the separation of medicinally active components by using selective solvents in standard extraction procedures. The products so obtained from plants are relatively impure liquids, semisolids or powders. These include classes of preparations known as maceration, decoctions, infusions, (Methods of Extraction of Medicinal Plants) Maceration
4 In this process, the drug is extracted with the solvent liquid between 2-14 days. Usually the process is carried out at C. The insoluble solid is removed by filtration. It is necessary to prevent evaporation of solvent during filtration. Infusion This is a form of maceration in which gentle heat is used during the process of extraction. It is used when moderately elevated temperature is not objectionable. The solvent efficiency of the menstruum is thereby increased. Every pot of coffee or cup of tea involves solid/liquid extraction, the extraction of organic compounds from solid ground beans or leaves using hot water as the liquid. The lower molecular weight polar molecules such as caffeine dissolve in the hot water and are removed from the high molecular weight water-insoluble cellulose, protein, and lipid materials. Decoction In this process, the crude drug is boiled in a specified volume of water for a defined time; it is then cooled and strained or filtered. This procedure is suitable for extracting water-soluble, heatstable constituents. The concentrated extract is filtered and used as such or processed further. Percolation This is the procedure used most frequently to extract active ingredients in the preparation of tinctures and fluid extracts. A percolator (a narrow, cone-shaped vessel open at both ends) is generally used. The solid ingredients are moistened with an appropriate amount of the specified menstruum and allowed to stand for approximately 4 h in a well closed container, after which the mass is packed and the top of the percolator is closed. Additional menstruum is added to form a shallow layer above the mass, and the mixture is allowed to macerate in the closed percolator for 24 h. The outlet of the percolator then is opened and the liquid contained therein is allowed to drip slowly. Additional menstruum is added as required, until the percolate measures about three-quarters of the required volume of the finished product. The marc is then pressed and the expressed liquid is added to the percolate. Sufficient menstruum is added to produce the required volume, and the mixed liquid is clarified by filtration or by standing followed by decanting. Hot Continuous Extraction (Soxhlet) In this method, the finely ground crude drug is placed in a porous bag or thimble made of strong filter paper, which is placed in chamber E of the Soxhlet apparatus (Figure 2). The extracting solvent in flask A is heated, and its vapors condense in condenser D. The condensed extractant drips into the thimble containing the crude drug, and extracts it by contact. When the level of liquid in chamber E rises to the top of siphon tube C, the liquid contents of chamber E siphon into fl ask A. This process is
5 continuous and is carried out until a drop of solvent from the siphon tube does not leave residue when evaporated. The advantage of this method, compared to previously described methods, is that large amounts of drug can be extracted with a much smaller quantity of solvent. This effects tremendous economy in terms of time, energy and consequently financial inputs. At small scale, it is employed as a batch process only, but it becomes much more economical and viable when converted into a continuous extraction procedure on medium or large scale.
Comparative determination of glycosides in senna by using different methods of extraction (Soxhlet, maceration and ultrasonic bath)
 1 Experiment 1, 2 and 3 Comparative determination of glycosides in senna by using different methods of extraction (Soxhlet, maceration and ultrasonic bath) Aim: determine the yield among different extraction
1 Experiment 1, 2 and 3 Comparative determination of glycosides in senna by using different methods of extraction (Soxhlet, maceration and ultrasonic bath) Aim: determine the yield among different extraction
Separation of a Mixture
 Separation of a Mixture The isolation of pure components of a mixture requires the separation of one component from another. Chemists have developed techniques for doing this. These methods take advantage
Separation of a Mixture The isolation of pure components of a mixture requires the separation of one component from another. Chemists have developed techniques for doing this. These methods take advantage
CHEM Experiment 4 Introduction to Separation Techniques I. Objectives
 1 CHEM 0011 Experiment 4 Introduction to Separation Techniques I Objectives 1. To learn the gravity filtration technique 2. To learn the suction filtration technique 3. To learn about solvent extraction
1 CHEM 0011 Experiment 4 Introduction to Separation Techniques I Objectives 1. To learn the gravity filtration technique 2. To learn the suction filtration technique 3. To learn about solvent extraction
The Separation of a Mixture into Pure Substances
 The Separation of a Mixture into Pure Substances The experiment is designed to familiarize you with some standard chemical techniques and to encourage careful work in separating and weighing chemicals.
The Separation of a Mixture into Pure Substances The experiment is designed to familiarize you with some standard chemical techniques and to encourage careful work in separating and weighing chemicals.
E25 ISOLATION OF A BIOLOGICALLY ACTIVE COMPOUND The isolation of caffeine from tea leaves
 E25 ISLATI F A BILGICALLY ACTIVE CMPUD The isolation of caffeine from tea leaves ITRDUCTI The overwhelmin majority of bioloically active molecules are oranic compounds, e.. alcohol, salicylic acid and
E25 ISLATI F A BILGICALLY ACTIVE CMPUD The isolation of caffeine from tea leaves ITRDUCTI The overwhelmin majority of bioloically active molecules are oranic compounds, e.. alcohol, salicylic acid and
Separating the Components of a Mixture
 Separating the Components of a Mixture Introduction: Mixtures are not unique to chemistry; we encounter them on a daily basis. The food and drinks we consume, the fuel we use in our vehicles, building
Separating the Components of a Mixture Introduction: Mixtures are not unique to chemistry; we encounter them on a daily basis. The food and drinks we consume, the fuel we use in our vehicles, building
Extraction of Caffeine From Coffee or Tea
 Extraction of Caffeine From Coffee or Tea Techniques Week ne Interpreting a Handbook (C 3) Extraction and Washing (C 15 & 37) Clamps and Clamping (C 19) Week Two Distillation (C20) Green Principles Less
Extraction of Caffeine From Coffee or Tea Techniques Week ne Interpreting a Handbook (C 3) Extraction and Washing (C 15 & 37) Clamps and Clamping (C 19) Week Two Distillation (C20) Green Principles Less
Synthesis 0732: Isolating Caffeine from Tea
 Work Completed: 01.22.09 Work Submitted: 02.03.09 Synthesis 0732: Isolating Caffeine from Tea Abstract Caffeine was extracted from instant tea and purified by recrystallization. The yield was determined
Work Completed: 01.22.09 Work Submitted: 02.03.09 Synthesis 0732: Isolating Caffeine from Tea Abstract Caffeine was extracted from instant tea and purified by recrystallization. The yield was determined
Separating the Components of a Mixture
 Separating the Components of a Mixture Introduction Many naturally occurring substances occur as mixtures rather than pure substances. There are two main types of mixtures, homogeneous and heterogeneous.
Separating the Components of a Mixture Introduction Many naturally occurring substances occur as mixtures rather than pure substances. There are two main types of mixtures, homogeneous and heterogeneous.
Separating the Components of a Mixture
 Separating the Components of a Mixture Introduction Many naturally occurring substances occur as mixtures rather than pure substances. There are two main types of mixtures, homogeneous and heterogeneous.
Separating the Components of a Mixture Introduction Many naturally occurring substances occur as mixtures rather than pure substances. There are two main types of mixtures, homogeneous and heterogeneous.
Experiment 3: Separation of a Mixture Pre-lab Exercise
 1 Experiment 3: Separation of a Mixture Pre-lab Exercise Name: The amounts of sand, salt, and benzoic acid that will dissolve in 100 g of water at different temperatures: Temperature 0 C 20 C 40 C 60 C
1 Experiment 3: Separation of a Mixture Pre-lab Exercise Name: The amounts of sand, salt, and benzoic acid that will dissolve in 100 g of water at different temperatures: Temperature 0 C 20 C 40 C 60 C
Student Handout Procedure
 Student Handout Procedure Lab period 1: Reaction: Measure 0.75 g of solid cinnamic acid and 25 ml of your unknown alcohol in a 100 ml round bottom flask. Add a stir bar and stir solution until it is completely
Student Handout Procedure Lab period 1: Reaction: Measure 0.75 g of solid cinnamic acid and 25 ml of your unknown alcohol in a 100 ml round bottom flask. Add a stir bar and stir solution until it is completely
Figure 11.1 Derivatives of Salicylic Acid O C OH OCH3. Na + OH sodium salicylate. OH CH3 Acetylsaliclic acid Aspirin.
 Experiment 11 heck-in; A. heck-in Be sure that all of your glassware is present in your locker at check-in time. nce you have checked-in you will be held responsible for missing or damaged glassware items.
Experiment 11 heck-in; A. heck-in Be sure that all of your glassware is present in your locker at check-in time. nce you have checked-in you will be held responsible for missing or damaged glassware items.
Filtering and evaporation
 Filtering and evaporation How can we get clean water? STARTER Match the equipment diagrams to the correct names. Beaker Evaporating Basin Pestle and Mortar Bung Conical Flask Spatula Pipette Measuring
Filtering and evaporation How can we get clean water? STARTER Match the equipment diagrams to the correct names. Beaker Evaporating Basin Pestle and Mortar Bung Conical Flask Spatula Pipette Measuring
Gravimetric Analysis
 Experiment 1: Gravimetric Analysis with Calcium Chloride and Potassium Carbonate In this experiment, proper analytical experimental techniques will be utilized to perform a double displacement reaction.
Experiment 1: Gravimetric Analysis with Calcium Chloride and Potassium Carbonate In this experiment, proper analytical experimental techniques will be utilized to perform a double displacement reaction.
Experiment 6 Thin-Layer Chromatography (TLC)
 Experiment 6 Thin-Layer Chromatography (TLC) OUTCOMES After completing this experiment, the student should be able to: explain basic principles of chromatography in general. describe important aspects
Experiment 6 Thin-Layer Chromatography (TLC) OUTCOMES After completing this experiment, the student should be able to: explain basic principles of chromatography in general. describe important aspects
Preparation 1: Chloroform
 SECTION 3: General Lab Procedures Part 3: The Preparation of General Lab Chemicals General laboratory processes involve those chemical reactions where basic chemicals are being reacted, and produced. General
SECTION 3: General Lab Procedures Part 3: The Preparation of General Lab Chemicals General laboratory processes involve those chemical reactions where basic chemicals are being reacted, and produced. General
15. Extraction: Isolation of Caffeine from Tea
 15. Extraction: Isolation of Caffeine from Tea In this experiment you will isolate a compound from a natural source using two extraction techniques. Such compounds are often referred to as natural products.
15. Extraction: Isolation of Caffeine from Tea In this experiment you will isolate a compound from a natural source using two extraction techniques. Such compounds are often referred to as natural products.
Prototocatechualdehyde methylenation. Photo-essay.
 Prototocatechualdehyde methylenation. Photo-essay. What follows is a slight variation of the commonly referenced catechol methylenation procedure, easily found copied and pasted all over the internet.
Prototocatechualdehyde methylenation. Photo-essay. What follows is a slight variation of the commonly referenced catechol methylenation procedure, easily found copied and pasted all over the internet.
Separations. Objective. Background. Date Lab Time Name
 Objective Separations Techniques of separating mixtures will be illustrated using chromatographic methods. The natural pigments found in spinach leaves, β-carotene and chlorophyll, will be separated using
Objective Separations Techniques of separating mixtures will be illustrated using chromatographic methods. The natural pigments found in spinach leaves, β-carotene and chlorophyll, will be separated using
2. Other constituents in the sample solution should not interfere with the precipitation of the component of interest.
 EXPERIMENT 15 Percentage Yield of Lead (II) Iodide in a Gravimetric Analysis INTRODUCTION In a gravimetric analysis, a substance is treated so that the component of interest is separated either in its
EXPERIMENT 15 Percentage Yield of Lead (II) Iodide in a Gravimetric Analysis INTRODUCTION In a gravimetric analysis, a substance is treated so that the component of interest is separated either in its
Lab 2. Drug Abuse. Solubility and Colligative Properties of Solutions: Coffee, Soda, and Ice Cream
 Lab 2. Drug Abuse. Solubility and Colligative Properties of Solutions: Coffee, Soda, and Ice Cream How do I make a stronger cup of coffee? How do I make ice cream? Prelab Spend 5 minutes doing the following
Lab 2. Drug Abuse. Solubility and Colligative Properties of Solutions: Coffee, Soda, and Ice Cream How do I make a stronger cup of coffee? How do I make ice cream? Prelab Spend 5 minutes doing the following
Lab 2. Drug Abuse. Solubility and Colligative Properties of Solutions: Coffee, Soda, and Ice Cream
 Lab 2. Drug Abuse. Solubility and Colligative Properties of Solutions: Coffee, Soda, and Ice Cream How do I make a stronger cup of coffee? How do I make ice cream? Prelab Spend 5 minutes doing the following
Lab 2. Drug Abuse. Solubility and Colligative Properties of Solutions: Coffee, Soda, and Ice Cream How do I make a stronger cup of coffee? How do I make ice cream? Prelab Spend 5 minutes doing the following
SYNTHESIS OF SALICYLIC ACID
 26 SYNTHESIS OF SALICYLIC ACID The purpose of this experiment is to synthesize salicylic acid, a white organic solid that was extracted from willow bark by Hippocrates in the fifth century BC. At that
26 SYNTHESIS OF SALICYLIC ACID The purpose of this experiment is to synthesize salicylic acid, a white organic solid that was extracted from willow bark by Hippocrates in the fifth century BC. At that
Coffee Filter Chromatography
 Here is a summary of what you will learn in this section: Solutions can be separated by filtration, paper chromatography, evaporation, or distillation. Mechanical mixtures can be separated by sorting,
Here is a summary of what you will learn in this section: Solutions can be separated by filtration, paper chromatography, evaporation, or distillation. Mechanical mixtures can be separated by sorting,
EXPERIMENT #3: Extraction and Drying Agents: Extraction of Caffeine from Tea
 EXPERIMENT #3: Extraction and Drying Agents: Extraction of Caffeine from Tea Chem 241, Lab Section In this experiment we will extract caffeine from tea leaves while learning several new laboratory techniques,
EXPERIMENT #3: Extraction and Drying Agents: Extraction of Caffeine from Tea Chem 241, Lab Section In this experiment we will extract caffeine from tea leaves while learning several new laboratory techniques,
LAB: One Tube Reaction Part 1
 AP Chemistry LAB: One Tube Reaction Part 1 Objective: To monitor and document the chemical changes occurring in a single test tube containing a predetermined mixture of chemicals. Materials: test tube,
AP Chemistry LAB: One Tube Reaction Part 1 Objective: To monitor and document the chemical changes occurring in a single test tube containing a predetermined mixture of chemicals. Materials: test tube,
Paper Chromatography and Steam Distillation of Orange Oil EVERY STUDENT MUST BRING AN ORANGE TO LAB FOR THIS EXPERIMENT! Equipment
 Paper Chromatography and Steam Distillation of Orange Oil EVERY STUDENT MUST BRING AN ORANGE TO LAB FOR THIS EXPERIMENT! Equipment You will need a 600 ml beaker, a 50 ml graduated cylinder, 4 Expo Wet
Paper Chromatography and Steam Distillation of Orange Oil EVERY STUDENT MUST BRING AN ORANGE TO LAB FOR THIS EXPERIMENT! Equipment You will need a 600 ml beaker, a 50 ml graduated cylinder, 4 Expo Wet
Analytical Method for Coumaphos (Targeted to agricultural, animal and fishery products)
 Analytical Method for Coumaphos (Targeted to agricultural, animal and fishery products) The target compound to be determined is coumaphos. 1. Instruments Gas chromatograph-flame thermionic detector (GC-FTD)
Analytical Method for Coumaphos (Targeted to agricultural, animal and fishery products) The target compound to be determined is coumaphos. 1. Instruments Gas chromatograph-flame thermionic detector (GC-FTD)
Sample Questions for the Chemistry of Coffee Topic Test
 Sample Questions for the Chemistry of Coffee Topic Test 1. During the 2013 Barista Championship, one of the contestants used a distillation apparatus to deliver a distilled coffee product as his specialty
Sample Questions for the Chemistry of Coffee Topic Test 1. During the 2013 Barista Championship, one of the contestants used a distillation apparatus to deliver a distilled coffee product as his specialty
Maceration Percolation And Infusion Techniques Of
 We have made it easy for you to find a PDF Ebooks without any digging. And by having access to our ebooks online or by storing it on your computer, you have convenient answers with maceration percolation
We have made it easy for you to find a PDF Ebooks without any digging. And by having access to our ebooks online or by storing it on your computer, you have convenient answers with maceration percolation
Distillation Purification of Liquids
 Distillation Purification of Liquids Types of Distillations commonly used in Organic Lab: Simple separates volatile compounds (liquids) from non-volatile compounds (solids) or volatiles with boiling points
Distillation Purification of Liquids Types of Distillations commonly used in Organic Lab: Simple separates volatile compounds (liquids) from non-volatile compounds (solids) or volatiles with boiling points
FAT, TOTAL (Hydrolysis)
 FATTO.01-1 FAT, TOTAL (Hydrolysis) PRINCIPLE The major portions of the native fats in corn starch are bound in a manner as to render them unextractable by the usual methods of solvent extraction. When
FATTO.01-1 FAT, TOTAL (Hydrolysis) PRINCIPLE The major portions of the native fats in corn starch are bound in a manner as to render them unextractable by the usual methods of solvent extraction. When
An Economic And Simple Purification Procedure For The Large-Scale Production Of Ovotransferrin From Egg White
 An Economic And Simple Purification Procedure For The Large-Scale Production Of Ovotransferrin From Egg White D. U. Ahn, E. J. Lee and A. Pometto Department of Animal Science, Iowa State University, Ames,
An Economic And Simple Purification Procedure For The Large-Scale Production Of Ovotransferrin From Egg White D. U. Ahn, E. J. Lee and A. Pometto Department of Animal Science, Iowa State University, Ames,
(a) Dead-end/conventional filtration fluid flow perpendicular to the filter medium. (b) Crossflow filtration fluid flow parallel to the filter
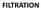 FILTRATION (a) Dead-end/conventional filtration fluid flow perpendicular to the filter medium. (b) Crossflow filtration fluid flow parallel to the filter medium. Filtration Generally carry out in the early
FILTRATION (a) Dead-end/conventional filtration fluid flow perpendicular to the filter medium. (b) Crossflow filtration fluid flow parallel to the filter medium. Filtration Generally carry out in the early
Molecular Gastronomy: The Chemistry of Cooking
 Molecular Gastronomy: The Chemistry of Cooking We re surrounded by chemistry each and every day but some instances are more obvious than others. Most people recognize that their medicine is the product
Molecular Gastronomy: The Chemistry of Cooking We re surrounded by chemistry each and every day but some instances are more obvious than others. Most people recognize that their medicine is the product
IAJPS 2016, 3 (8), T. Balakrishna et al ISSN Available online at:
 CODEN (USA): IAJPBB ISSN: 2349-7750 INDO AMERICAN JOURNAL OF PHARMACEUTICAL SCIENCES Available online at: http://www.iajps.com Review Article A REVIEW ON EXTRACTION TECHNIQUES T. Balakrishna*, S. Vidyadhara,
CODEN (USA): IAJPBB ISSN: 2349-7750 INDO AMERICAN JOURNAL OF PHARMACEUTICAL SCIENCES Available online at: http://www.iajps.com Review Article A REVIEW ON EXTRACTION TECHNIQUES T. Balakrishna*, S. Vidyadhara,
CHEM 321: EXTRACTION AND PURIFICATION OF CAFFEINE
 CHEM 321: EXTRACTI AD PURIFICATI F CAFFEIE Structurally complex organic chemicals sometimes can be isolated easily from natural sources. More often, a complex compound of interest (an insect pheromone
CHEM 321: EXTRACTI AD PURIFICATI F CAFFEIE Structurally complex organic chemicals sometimes can be isolated easily from natural sources. More often, a complex compound of interest (an insect pheromone
GB Translated English of Chinese Standard: GB NATIONAL STANDARD
 Translated English of Chinese Standard: GB5009.6-2016 www.chinesestandard.net Sales@ChineseStandard.net GB NATIONAL STANDARD OF THE PEOPLE S REPUBLIC OF CHINA GB 5009.6-2016 National food safety standard
Translated English of Chinese Standard: GB5009.6-2016 www.chinesestandard.net Sales@ChineseStandard.net GB NATIONAL STANDARD OF THE PEOPLE S REPUBLIC OF CHINA GB 5009.6-2016 National food safety standard
Pharmaceutical Compounding
 Pharmaceutical Compounding Preparation of Syrup, Spirit and Elixir Hemin J Majeed MSc. Pharmaceutical sciences 1 Syrup Syrups: Are sweet, viscous aqueous liquids, they are concentrated aqueous preparations
Pharmaceutical Compounding Preparation of Syrup, Spirit and Elixir Hemin J Majeed MSc. Pharmaceutical sciences 1 Syrup Syrups: Are sweet, viscous aqueous liquids, they are concentrated aqueous preparations
Avocado. recipe or working method? WLODEK. Wlodzimierz S. BOREJSZA-WYSOCKI Ph.D. IR-4 Southern Regional Laboratory Research Director
 Avocado recipe or working method? WLODEK Wlodzimierz S. BOREJSZA-WYSOCKI Ph.D. IR-4 Southern Regional Laboratory Research Director Food & Environmental Toxicology Laboratory Institute of Food and Agricultural
Avocado recipe or working method? WLODEK Wlodzimierz S. BOREJSZA-WYSOCKI Ph.D. IR-4 Southern Regional Laboratory Research Director Food & Environmental Toxicology Laboratory Institute of Food and Agricultural
The Lazy Mans Guide to Extracting Mimosa Hostilis Root Bark by Vortex A report and guide for a new way of extracting MHRB
 The Lazy Mans Guide to Extracting Mimosa Hostilis Root Bark by Vortex A report and guide for a new way of extracting MHRB Extraction Time: 1 gm in 2.5 hrs, 4 gm in 7 hr, 7.5 g total @48 hrs Equipment:
The Lazy Mans Guide to Extracting Mimosa Hostilis Root Bark by Vortex A report and guide for a new way of extracting MHRB Extraction Time: 1 gm in 2.5 hrs, 4 gm in 7 hr, 7.5 g total @48 hrs Equipment:
Sequential Separation of Lysozyme, Ovomucin, Ovotransferrin and Ovalbumin from Egg White
 AS 662 ASL R3104 2016 Sequential Separation of Lysozyme, Ovomucin, Ovotransferrin and Ovalbumin from Egg White Sandun Abeyrathne Iowa State University Hyunyong Lee Iowa State University, hdragon@iastate.edu
AS 662 ASL R3104 2016 Sequential Separation of Lysozyme, Ovomucin, Ovotransferrin and Ovalbumin from Egg White Sandun Abeyrathne Iowa State University Hyunyong Lee Iowa State University, hdragon@iastate.edu
Natural Oil Preparation and Processing
 Natural Oil Preparation and Processing Tony O Lenick Siltech LLC Oil extracted from the pressing of seeds contains many ingredients, some desirable and others undesirable. Crude oil is processed to separate
Natural Oil Preparation and Processing Tony O Lenick Siltech LLC Oil extracted from the pressing of seeds contains many ingredients, some desirable and others undesirable. Crude oil is processed to separate
Determination of caffeine content in tea and soft drink. BCH445 [Practical] 1
![Determination of caffeine content in tea and soft drink. BCH445 [Practical] 1 Determination of caffeine content in tea and soft drink. BCH445 [Practical] 1](/thumbs/95/124814804.jpg) Determination of caffeine content in tea and soft drink BCH445 [Practical] 1 Caffeine, the common name for 1,3,7-trimethylxanthine. It belongs to a group of methylxanthene. 2 Caffeine is a chemical that
Determination of caffeine content in tea and soft drink BCH445 [Practical] 1 Caffeine, the common name for 1,3,7-trimethylxanthine. It belongs to a group of methylxanthene. 2 Caffeine is a chemical that
The grade 5 English science unit, Solutions, meets the academic content standards set in the Korean curriculum, which state students should:
 This unit deals with how solids dissolve in liquids and what affects their dissolution. By studying the dissolution process and related factors, students develop an interest in and curiosity about solutions.
This unit deals with how solids dissolve in liquids and what affects their dissolution. By studying the dissolution process and related factors, students develop an interest in and curiosity about solutions.
C27 Chromatography. Collect: Column Mortar and pestle Dropper (229 mm) Capillary tube TLC plate Aluminum foil UV light
 C27 Chromatography (2017/04/24) Collect: Column Mortar and pestle Dropper (229 mm) Capillary tube TLC plate Aluminum foil UV light Prepare: Green leaves Beaker (30 100 ml) Erlenmeyer flask (50, 125 ml)
C27 Chromatography (2017/04/24) Collect: Column Mortar and pestle Dropper (229 mm) Capillary tube TLC plate Aluminum foil UV light Prepare: Green leaves Beaker (30 100 ml) Erlenmeyer flask (50, 125 ml)
THIN LAYER CHROMATOGRAPHY AND MELTING POINT DETERMINATION: DETECTION OF CAFFEINE IN VARIOUS SAMPLES
 EXPERIMENT 8 THIN LAYER CHROMATOGRAPHY AND MELTING POINT DETERMINATION: DETECTION OF CAFFEINE IN VARIOUS SAMPLES Additional Resources http://orgchem.colorado.edu/hndbksupport/tlc/tlc.html http://coffeefaq.com/caffaq.html
EXPERIMENT 8 THIN LAYER CHROMATOGRAPHY AND MELTING POINT DETERMINATION: DETECTION OF CAFFEINE IN VARIOUS SAMPLES Additional Resources http://orgchem.colorado.edu/hndbksupport/tlc/tlc.html http://coffeefaq.com/caffaq.html
Pharmacognosy- 1 PHG 222. Prof. Dr. Amani S. Awaad
 Pharmacognosy- 1 PHG 222 Prof. Dr. Amani S. Awaad Professor of Pharmacognosy Pharmacognosy Department, College of Pharmacy Salman Bin Abdulaziz University, Al-Kharj. KSA. Email: amaniawaad@hotmail.com
Pharmacognosy- 1 PHG 222 Prof. Dr. Amani S. Awaad Professor of Pharmacognosy Pharmacognosy Department, College of Pharmacy Salman Bin Abdulaziz University, Al-Kharj. KSA. Email: amaniawaad@hotmail.com
Practical 1 - Determination of Quinine in Tonic Water
 Practical 1 - Determination of Quinine in Tonic Water Introduction Quinine has a fluorescence and a UV absorbance and so can be quantified using either of these. In the method described here the absorbances
Practical 1 - Determination of Quinine in Tonic Water Introduction Quinine has a fluorescence and a UV absorbance and so can be quantified using either of these. In the method described here the absorbances
Analysis of tea powder for adulterant
 IOSR Journal of Pharmacy and Biological Sciences (IOSR-JPBS) e-issn:2278-3008, p-issn:2319-7676. Volume 12, Issue 4 Ver. VI (Jul Aug 2017), PP 37-42 www.iosrjournals.org Analysis of tea powder for adulterant
IOSR Journal of Pharmacy and Biological Sciences (IOSR-JPBS) e-issn:2278-3008, p-issn:2319-7676. Volume 12, Issue 4 Ver. VI (Jul Aug 2017), PP 37-42 www.iosrjournals.org Analysis of tea powder for adulterant
National Food Safety Standard
 Translated English of Chinese Standard: GB 5413.30-2010 www.chinesestandard.net Email: Sales@ChineseStandard.net GB NATIONAL STANDARD OF THE PEOPLE S REPUBLIC OF CHINA GB 5413.30-2010 National Food Safety
Translated English of Chinese Standard: GB 5413.30-2010 www.chinesestandard.net Email: Sales@ChineseStandard.net GB NATIONAL STANDARD OF THE PEOPLE S REPUBLIC OF CHINA GB 5413.30-2010 National Food Safety
3. Aspirin Analysis. Prelaboratory Assignment. 3.1 Introduction
 In this experiment, you will analyze the purity of your crude and recrystallized aspirin products using a method called thin layer chromatography (TLC). You will also determine the percent yield of your
In this experiment, you will analyze the purity of your crude and recrystallized aspirin products using a method called thin layer chromatography (TLC). You will also determine the percent yield of your
UNIT 2 Production of natural drug products
 Production of natural drug products 5 UNIT 2 Production of natural drug products 1. Collection (wild) 2. Cultivation (commercial), collection, harvesting, drying, garbling, packaging, storage and preservation
Production of natural drug products 5 UNIT 2 Production of natural drug products 1. Collection (wild) 2. Cultivation (commercial), collection, harvesting, drying, garbling, packaging, storage and preservation
Chapter 5 SEPARATION OF SUBSTANCES
 Chapter 5 SEPARATION OF SUBSTANCES Subjective Type Exercises A. Very Short Answer Questions 1. We observe different instances of separation of materials. How will you separate the following? (a) Tea leaves
Chapter 5 SEPARATION OF SUBSTANCES Subjective Type Exercises A. Very Short Answer Questions 1. We observe different instances of separation of materials. How will you separate the following? (a) Tea leaves
Royal Society of Chemistry Analytical Division East Anglia Region National Schools' Analyst Competition
 Royal Society of Chemistry Analytical Division East Anglia Region 2017 National Schools' Analyst Competition East Anglia Region Heat Thursday 20th April, 2017 School of Chemistry University of East Anglia
Royal Society of Chemistry Analytical Division East Anglia Region 2017 National Schools' Analyst Competition East Anglia Region Heat Thursday 20th April, 2017 School of Chemistry University of East Anglia
Extraction of Acrylamide from Coffee Using ISOLUTE. SLE+ Prior to LC-MS/MS Analysis
 Application Note AN796 Extraction of Acrylamide from Coffee using ISOLUTE SLE+ Page 1 Extraction of Acrylamide from Coffee Using ISOLUTE SLE+ Prior to LC-MS/MS Analysis This application note describes
Application Note AN796 Extraction of Acrylamide from Coffee using ISOLUTE SLE+ Page 1 Extraction of Acrylamide from Coffee Using ISOLUTE SLE+ Prior to LC-MS/MS Analysis This application note describes
7.2.4 Mixtures. 100 minutes. 146 marks. Page 1 of 42
 7.2.4 Mixtures 100 minutes 146 marks Page 1 of 42 ## John ground some coffee beans into little pieces. He put them into a coffee filter and poured 800 cm 3 of boiling water over them to make a jug of coffee.
7.2.4 Mixtures 100 minutes 146 marks Page 1 of 42 ## John ground some coffee beans into little pieces. He put them into a coffee filter and poured 800 cm 3 of boiling water over them to make a jug of coffee.
involves separating solid in liquid mixtures where the solid particles are large, such as vegetables in water, where you want to retrieve the solid.
 A mixture is formed when two or more substances are mixed physically and not chemically combined. These substances can be separated and recovered again by physical and not chemical means, although not
A mixture is formed when two or more substances are mixed physically and not chemically combined. These substances can be separated and recovered again by physical and not chemical means, although not
Application Note No. 184/2015
 Application Note No. 184/2015 Fat determination in Yogurt Extraction Unit E-816 ECE: Fat Determination in Yogurt samples using Twisselmann and Soxhlet extraction www.buchi.com Quality in your hands 1.
Application Note No. 184/2015 Fat determination in Yogurt Extraction Unit E-816 ECE: Fat Determination in Yogurt samples using Twisselmann and Soxhlet extraction www.buchi.com Quality in your hands 1.
Background information Year 7, unit 1: Mixing and separating
 Background information Year 7, unit 1: Mixing and separating Mixtures When two or more kinds of matter are put together a mixture is formed. The process of being mixed does not change the components of
Background information Year 7, unit 1: Mixing and separating Mixtures When two or more kinds of matter are put together a mixture is formed. The process of being mixed does not change the components of
Dispensing Techniques
 Dispensing Techniques Compounding and Good Practice Compounding (Extemporaneous Dispensing) Definition: A small-scale manufacture of medicines from basic ingredients in the community or in hospital pharmacy
Dispensing Techniques Compounding and Good Practice Compounding (Extemporaneous Dispensing) Definition: A small-scale manufacture of medicines from basic ingredients in the community or in hospital pharmacy
Determination of Alcohol Content of Wine by Distillation followed by Density Determination by Hydrometry
 Sirromet Wines Pty Ltd 850-938 Mount Cotton Rd Mount Cotton Queensland Australia 4165 www.sirromet.com Courtesy of Jessica Ferguson Assistant Winemaker & Chemist Downloaded from seniorchem.com/eei.html
Sirromet Wines Pty Ltd 850-938 Mount Cotton Rd Mount Cotton Queensland Australia 4165 www.sirromet.com Courtesy of Jessica Ferguson Assistant Winemaker & Chemist Downloaded from seniorchem.com/eei.html
Properties of Water Lab: What Makes Water Special? An Investigation of the Liquid That Makes All Life Possible: Water!
 Properties of Water Lab: What Makes Water Special? An Investigation of the Liquid That Makes All Life Possible: Water! Background: Water has some peculiar properties, but because it is the most common
Properties of Water Lab: What Makes Water Special? An Investigation of the Liquid That Makes All Life Possible: Water! Background: Water has some peculiar properties, but because it is the most common
1. What is made when a solute is dissolved in a solvent?
 A solution is made when a solute dissolves in a solvent. The solutions we will look at are those where a solid dissolves in a liquid. The solid is the solute and the liquid is the solvent. Solute + Solvent
A solution is made when a solute dissolves in a solvent. The solutions we will look at are those where a solid dissolves in a liquid. The solid is the solute and the liquid is the solvent. Solute + Solvent
Chapter 14 Tex-619-J, Analysis of Water for Chloride and Sulfate Ions
 Chapter 14 Tex-619-J, Analysis of Water for Contents: Section 1 Overview... 14-2 Section 2 Apparatus... 14-3 Section 3 Reagents... 14-4 Section 4 Procedures... 14-5 Section 5 Calculations... 14-6 Section
Chapter 14 Tex-619-J, Analysis of Water for Contents: Section 1 Overview... 14-2 Section 2 Apparatus... 14-3 Section 3 Reagents... 14-4 Section 4 Procedures... 14-5 Section 5 Calculations... 14-6 Section
2.8 Bentonite fining. Chapter: Clarification page 19 of 38
 page 19 of 38 2.8 Bentonite fining Bentonite fining is chiefly carried out to stabilize beverages against protein hazes. Grapes have a relatively high content of natural protein compared to other fruits.
page 19 of 38 2.8 Bentonite fining Bentonite fining is chiefly carried out to stabilize beverages against protein hazes. Grapes have a relatively high content of natural protein compared to other fruits.
In the preparation of this Tanzania Standard assistance was derived from:
 TANZANIA BUREAU OF STANDARDS DRAFT TANZANIA STANDARD COCONUT MILK AND COCONUT CREAM SPECIFICATION (DRAFT FOR COMMENT ONLY) AFDC 4 (3761) P3 0 FOREWORD Coconut milk and coconut cream shall be prepared by
TANZANIA BUREAU OF STANDARDS DRAFT TANZANIA STANDARD COCONUT MILK AND COCONUT CREAM SPECIFICATION (DRAFT FOR COMMENT ONLY) AFDC 4 (3761) P3 0 FOREWORD Coconut milk and coconut cream shall be prepared by
I. INTRODUCTION I ITEMS:
 Experiment 4 Chem 110 Lab LABORATORY TECHNIQUES PURPOSE: The purpose of this laboratory exercise is to develop safe laboratory skill and practice several laboratory techniques that will be used in many
Experiment 4 Chem 110 Lab LABORATORY TECHNIQUES PURPOSE: The purpose of this laboratory exercise is to develop safe laboratory skill and practice several laboratory techniques that will be used in many
Diffusion & Osmosis Labs
 AP Biology Diffusion & Osmosis Labs INTRODUCTION The life of a cell is dependent on efficiently moving material into and out of the cell across the cell membrane. All cells need sugars and oxygen to make
AP Biology Diffusion & Osmosis Labs INTRODUCTION The life of a cell is dependent on efficiently moving material into and out of the cell across the cell membrane. All cells need sugars and oxygen to make
COFFEE BASICS SCAA. The Elements of Proper Brewing and Creating an Ideal Coffee Drinking Experience
 COFFEE BASICS The Elements of Proper Brewing and Creating an Ideal Coffee Drinking Experience SCAA WATER THE ELEMENTS OF PROPER BREWING Fresh, good-tasting water is essential since it makes up more than
COFFEE BASICS The Elements of Proper Brewing and Creating an Ideal Coffee Drinking Experience SCAA WATER THE ELEMENTS OF PROPER BREWING Fresh, good-tasting water is essential since it makes up more than
PECTINASE Product Code: P129
 PECTINASE Product Code: P129 Enzyme for sample clarification prior to patulin analysis. For in vitro use only. P129/V1/02.06.16 www.r-biopharm.com Contents Page Test Principle... 3 Kit Components... 3
PECTINASE Product Code: P129 Enzyme for sample clarification prior to patulin analysis. For in vitro use only. P129/V1/02.06.16 www.r-biopharm.com Contents Page Test Principle... 3 Kit Components... 3
General overview of the two stages of the QuEChERS technique. Stage 1: Sample extraction. Stage 2: Sample cleanup
 QuEChERS Sample Preparation Procedures cat.# 25847, 25848, 25849, 25850, 25851, 25852, 26123, 26124, 26125, 26126, 26215, 26216, 26217, 26218, 26219, 26220, 26221, 26222, 26223, 26224, 26225, 26226, 26242,
QuEChERS Sample Preparation Procedures cat.# 25847, 25848, 25849, 25850, 25851, 25852, 26123, 26124, 26125, 26126, 26215, 26216, 26217, 26218, 26219, 26220, 26221, 26222, 26223, 26224, 26225, 26226, 26242,
MILK ADULTERATION. By, Gautami Shirsat Grisha Dialani Sushmita Suman
 MILK ADULTERATION By, Gautami Shirsat Grisha Dialani Sushmita Suman CONSUMER SURVEY Average consumption per day 1 lit. Type of consumption Directly as milk or in tea Mostly preferred Buffalo Milk Consumers
MILK ADULTERATION By, Gautami Shirsat Grisha Dialani Sushmita Suman CONSUMER SURVEY Average consumption per day 1 lit. Type of consumption Directly as milk or in tea Mostly preferred Buffalo Milk Consumers
DETERMINATION OF CAFFEINE IN TEA SAMPLES. Know how much caffeine you are Taking in with each cup of tea!
 DETERMINATION OF CAFFEINE IN TEA SAMPLES Know how much caffeine you are Taking in with each cup of tea! CONTENTS 1. Introduction 2. Theory 3. Uses of Caffeine 4. Effects of Caffeine 5. Procedure 6. Observations
DETERMINATION OF CAFFEINE IN TEA SAMPLES Know how much caffeine you are Taking in with each cup of tea! CONTENTS 1. Introduction 2. Theory 3. Uses of Caffeine 4. Effects of Caffeine 5. Procedure 6. Observations
Lab 2: Phase transitions & ice cream
 Lab 2: Phase transitions & ice cream Lab sections on Tuesday Sept 18 Friday Sept 21 In this lab you will observe how changing two parameters, pressure and salt concentration, affects the two phase transitions
Lab 2: Phase transitions & ice cream Lab sections on Tuesday Sept 18 Friday Sept 21 In this lab you will observe how changing two parameters, pressure and salt concentration, affects the two phase transitions
Downloaded from
 1M Separation of Substances 1. The strainer to strain tea from tea leaves acts as a (A) Filter(B) Condenser(C) Boiler(D) Churner 2. A mixture of sand and dried leaves can be separated by - (A) Magnetic
1M Separation of Substances 1. The strainer to strain tea from tea leaves acts as a (A) Filter(B) Condenser(C) Boiler(D) Churner 2. A mixture of sand and dried leaves can be separated by - (A) Magnetic
Investigating solutions
 Investigating solutions Part A: saturated solutions Sugar dissolved in water is an important component of soft drinks. You are going to investigate just how much sugar can be dissolved in water. sugar
Investigating solutions Part A: saturated solutions Sugar dissolved in water is an important component of soft drinks. You are going to investigate just how much sugar can be dissolved in water. sugar
Rose Water Distillation
 LUMAT 1(2), 2013 Rose Water Distillation Milja Helenius Unit of Chemistry Teacher Education, Department of Chemistry, University of Helsinki, Finland milja.helenius@helsinki.fi Maija Aksela Unit of Chemistry
LUMAT 1(2), 2013 Rose Water Distillation Milja Helenius Unit of Chemistry Teacher Education, Department of Chemistry, University of Helsinki, Finland milja.helenius@helsinki.fi Maija Aksela Unit of Chemistry
Anaerobic Cell Respiration by Yeast
 25 Marks (I) Anaerobic Cell Respiration by Yeast BACKGROUND: Yeast are tiny single-celled (unicellular) fungi. The organisms in the Kingdom Fungi are not capable of making their own food. Fungi, like any
25 Marks (I) Anaerobic Cell Respiration by Yeast BACKGROUND: Yeast are tiny single-celled (unicellular) fungi. The organisms in the Kingdom Fungi are not capable of making their own food. Fungi, like any
Supporting information for J. Med. Chem., 1992, 35(16), , DOI: /jm00094a025 BLOOM
 Supporting information for J. Med. Chem., 1992, 35(16), 3081 3084, DOI: 10.1021/jm00094a025 BLOOM 3081-3084 Terms & Conditions Electronic Supporting Information files are available without a subscription
Supporting information for J. Med. Chem., 1992, 35(16), 3081 3084, DOI: 10.1021/jm00094a025 BLOOM 3081-3084 Terms & Conditions Electronic Supporting Information files are available without a subscription
7.2.6 Filtration, Chromatography and Distillation
 7.2.6 Filtration, Chromatography and Distillation 121 minutes 179 marks Page 1 of 51 Q1. The following diagrams show two methods of separating substances. (a) What is the name of each method? Method 1
7.2.6 Filtration, Chromatography and Distillation 121 minutes 179 marks Page 1 of 51 Q1. The following diagrams show two methods of separating substances. (a) What is the name of each method? Method 1
Attention is drawn to the following places, which may be of interest for search:
 A23F COFFEE; TEA; THEIR SUBSTITUTES; MANUFACTURE, PREPARATION, OR INFUSION THEREOF (coffee or tea pots A47G 19/14; tea infusers A47G 19/16; apparatus for making beverages, e.g. coffee or tea, A47J 31/00;
A23F COFFEE; TEA; THEIR SUBSTITUTES; MANUFACTURE, PREPARATION, OR INFUSION THEREOF (coffee or tea pots A47G 19/14; tea infusers A47G 19/16; apparatus for making beverages, e.g. coffee or tea, A47J 31/00;
Separation of Ovotransferrin and Ovomucoid from Chicken Egg White
 Animal Industry Report AS 662 ASL R3105 2016 Separation of and from Chicken Egg White Sandun Abeyrathne Iowa State University Hyunyong Lee Iowa State University, hdragon@iastate.edu Dong U. Ahn Iowa State
Animal Industry Report AS 662 ASL R3105 2016 Separation of and from Chicken Egg White Sandun Abeyrathne Iowa State University Hyunyong Lee Iowa State University, hdragon@iastate.edu Dong U. Ahn Iowa State
Frequently Asked Questions
 Frequently Asked Questions As you begin to make your own spirits you will have questions. Here are the answers to some of the most common questions we are asked. Is the spirit making process natural? Yes.
Frequently Asked Questions As you begin to make your own spirits you will have questions. Here are the answers to some of the most common questions we are asked. Is the spirit making process natural? Yes.
The Premium Benefits of Steam Infusion UHT Treatment
 EDITORIAL October 2012 The Premium Benefits of Steam Infusion UHT Treatment UHT, or Ultra High Temperature, treatment uses high temperature for a short time to kill micro-organisms in a food or beverage
EDITORIAL October 2012 The Premium Benefits of Steam Infusion UHT Treatment UHT, or Ultra High Temperature, treatment uses high temperature for a short time to kill micro-organisms in a food or beverage
EXTRACTION PROCEDURE
 SPE Application Note for Multiresidue Exraction and Clean Up from Fruit and Vegetables This note outlines solid phase extraction (SPE) methodology for the multiresidue extraction and clean up of fruits
SPE Application Note for Multiresidue Exraction and Clean Up from Fruit and Vegetables This note outlines solid phase extraction (SPE) methodology for the multiresidue extraction and clean up of fruits
Benchtop Evaluations of Tampering with Pharmaceutical Dosage Forms
 Benchtop Evaluations of Tampering with Pharmaceutical Dosage Forms Opioid Abuse Resistance Conference Philip A. Goliber, Ph.D. Andrew s Enterprises, LLC October 5 Tampering To interfere so as to weaken
Benchtop Evaluations of Tampering with Pharmaceutical Dosage Forms Opioid Abuse Resistance Conference Philip A. Goliber, Ph.D. Andrew s Enterprises, LLC October 5 Tampering To interfere so as to weaken
CITRUS & ALLIED GLOSSARY OF INDUSTRY TERMS
 Flavorings are used to augment the innate flavor of food and beverage products and, in some cases, are used to provide the entire flavor of the product. Some examples of foods and beverages that use flavors
Flavorings are used to augment the innate flavor of food and beverage products and, in some cases, are used to provide the entire flavor of the product. Some examples of foods and beverages that use flavors
Investigation of the Solubility
 Part 1 Purpose The purpose of this part of the lab is to determine how temperature affects solubility. What factors affect solubility? You will observe individual sugar cubes dissolving in water at different
Part 1 Purpose The purpose of this part of the lab is to determine how temperature affects solubility. What factors affect solubility? You will observe individual sugar cubes dissolving in water at different
1. Blender: Osterizer, 10-speed, or equivalent. 2. Separatory Funnel: Kilborn or equivalent (see figure 1) 2. HCl Solution: HCl/water (7:93 by volume)
 EXTER.01-1 INFESTATION IN WHOLE CORN PRINCIPLE Whole corn is suspended in aqueous borax solution to float insects and insect fragments, which are collected on filter paper for microscopic identification
EXTER.01-1 INFESTATION IN WHOLE CORN PRINCIPLE Whole corn is suspended in aqueous borax solution to float insects and insect fragments, which are collected on filter paper for microscopic identification
Winemaking and Sulfur Dioxide
 Winemaking and Sulfur Dioxide Prepared and Presented by: Frank Schieber, Amateur Winemaker MoundTop MicroVinification Vermillion, SD www.moundtop.com schieber@usd.edu Outline: Sulfur Dioxide (Free SO 2
Winemaking and Sulfur Dioxide Prepared and Presented by: Frank Schieber, Amateur Winemaker MoundTop MicroVinification Vermillion, SD www.moundtop.com schieber@usd.edu Outline: Sulfur Dioxide (Free SO 2
Science Project for ICCE General Level
 Science Project for ICCE General Level Investigation into the distribution in foodstuffs and health benefits of Vitamin C Vitamin C is an important vitamin long associated with good health. In this project
Science Project for ICCE General Level Investigation into the distribution in foodstuffs and health benefits of Vitamin C Vitamin C is an important vitamin long associated with good health. In this project
icbse.com Ankit Bahuguna (Name and signature of the student)
 1 Know How much caffeine you are taking in with each cup of tea! Project Prepared By: Ankit Bahuguna XII-A Roll Number: Lovely Public Sr. Sec. School (P.D. Vihar) 2 First of all I would like to thank my
1 Know How much caffeine you are taking in with each cup of tea! Project Prepared By: Ankit Bahuguna XII-A Roll Number: Lovely Public Sr. Sec. School (P.D. Vihar) 2 First of all I would like to thank my
Islamic Kasim Tuet Memorial Secondary School. Chun Suk Kwan 6S (6)
 Islamic Kasim Tuet Memorial Secondary School Chun Suk Kwan 6S (6) Introduction Aim Principal of experiment Apparatus and Chemicals Procedure Precaution Result Discussion Conclusion References Acknowledgement
Islamic Kasim Tuet Memorial Secondary School Chun Suk Kwan 6S (6) Introduction Aim Principal of experiment Apparatus and Chemicals Procedure Precaution Result Discussion Conclusion References Acknowledgement
COFFEE BASICS. The Elements of Proper Brewing and Creating an Ideal Coffee Drinking Experience SCAA
 COFFEE BASICS The Elements of Proper Brewing and Creating an Ideal Coffee Drinking Experience SCAA WATER THE ELEMENTS OF Proper BREWING Fresh, good-tasting water is essential since it makes up more than
COFFEE BASICS The Elements of Proper Brewing and Creating an Ideal Coffee Drinking Experience SCAA WATER THE ELEMENTS OF Proper BREWING Fresh, good-tasting water is essential since it makes up more than
Rapid Analysis of Soft Drinks Using the ACQUITY UPLC H-Class System with the Waters Beverage Analysis Kit
 Rapid Analysis of Soft Drinks Using the ACQUITY UPLC H-Class System with the Waters Beverage Analysis Kit Mark E. Benvenuti, Raymond Giska, and Jennifer A. Burgess Waters Corporation, Milford, MA U.S.
Rapid Analysis of Soft Drinks Using the ACQUITY UPLC H-Class System with the Waters Beverage Analysis Kit Mark E. Benvenuti, Raymond Giska, and Jennifer A. Burgess Waters Corporation, Milford, MA U.S.
Characterization of Gum from Durian Seed and Application in Ice Cream
 Available online http://www.ijat-aatsea.com ISSN 1686-9141 Characterization of Gum from Durian Seed and Application in Ice Cream Jiraporn Sawasdikarn *, Waritchon Nilanon and Yardrung Suwannarat Faculty
Available online http://www.ijat-aatsea.com ISSN 1686-9141 Characterization of Gum from Durian Seed and Application in Ice Cream Jiraporn Sawasdikarn *, Waritchon Nilanon and Yardrung Suwannarat Faculty
HYDROGEN SULPHIDE FORMATION IN FERMENTING TODDY*
 Ceylon Cocon. Q. (1974) 25, 153-159 Printed in Sri Lanka. HYDROGEN SULPHIDE FORMATION IN FERMENTING TODDY* E. R. JANSZ, E. E. JEYARAJ, I. G. PREMARATNE and D. J. ABEYRATNE Industrial Microbiology Section,
Ceylon Cocon. Q. (1974) 25, 153-159 Printed in Sri Lanka. HYDROGEN SULPHIDE FORMATION IN FERMENTING TODDY* E. R. JANSZ, E. E. JEYARAJ, I. G. PREMARATNE and D. J. ABEYRATNE Industrial Microbiology Section,
Cooking with Alcohol
 Cooking with Alcohol Model 1. Ethanol is a simple organic carbon compound with the hydroxyl group bound to a carbon. Other alcohols include methanol and propanol (1- propanol or 2- propanol). Each of these
Cooking with Alcohol Model 1. Ethanol is a simple organic carbon compound with the hydroxyl group bound to a carbon. Other alcohols include methanol and propanol (1- propanol or 2- propanol). Each of these
