Unit 8: When Chemicals Meet Water The Properties of Solutions 1
|
|
|
- Darrell O’Brien’
- 6 years ago
- Views:
Transcription
1 Unit 8: When Chemicals Meet Water The Properties of Solutions 1 [Tease] Chemistry: Challenges and Solutions Unit 8: When Chemicals Meet Water The Properties of Solutions Hosted by Adam Brunet A chemical reaction happens when molecules collide. A reaction can take place between molecules that are in the solid state, but two substances can react much more quickly if all the molecules can get at each other at the same time. This happens when two gases are mixed together. Or two liquids. But it can also happen if solid materials are dissolved in a liquid solvent. And the most common solvent is water. ETHAN MILLER: We start with our water in our kettle. At a really high temperature you re going to get things that are more soluble and at lower temperatures you are going to be removing things that are less soluble. And at the end of that we are going to get a delicious cup of coffee. A wide range of compounds can dissolve in water and make what we call aqueous solutions, like the salty ocean. WHITMAN MILLER: The real problem that we are facing through ocean acidification is that the gas solution from the atmosphere actually mixes with the open ocean, and as it does that, it changes the chemistry of the ocean. Chemical reactions are the essence of life around us and the majority of them happen in solution. [Title: Unit 8 When Chemicals Meet Water] ADAM BRUNET: What is a solution? You might think of a liquid mixture like gasoline or shampoo, or a sugary sports drink. But solutions do not have to be liquids. They can be solids like the steel frame of a bike, which is a mixture of iron and carbon. Or one of the most important solutions in the world the air we breathe. But there s a special place in chemistry for solutions that use water because a majority of solutions that affect us daily, like the blood running through our bodies or the liquid in the vascular system of these trees, all consist of water solutions. [SEGMENT 1: What is a Solution?] ADAM BRUNET: Hi, my name s Adam Brunet, and I m a chemistry professor. I have here the makings of a simple solution. A bottle of H 2 O, water, and a powdered sports drink, which is mostly salt and sugar.
2 Unit 8: When Chemicals Meet Water The Properties of Solutions 2 Now the water in the bottle is not yet a solution, because it consists of only one kind of molecule: H 2 O. But what happens when we add the powder? The water in this mixture is called the solvent because there is more of it than any of the substances in the powder. The solids in the powder are called solutes. At first, they settle to the bottom of the bottle, so it s not a uniform mixture. If I were to take a taste from the top, I would find that it s very watery. If I were to take a taste from the bottom of the bottle, I would find it to be very sugary and sweet. After a little while, however, the solids will have dissolved and spread evenly through the water. You don t have to shake it to make it dissolve, but I m going to speed things up a little. Now, if I sampled the water anywhere in the container, it has the same amount of dissolved solids throughout, and it would taste the same. We can say that the mixture is homogenous. Now we can call the mixture a solution. [SEGMENT 2: The Coffee Solution] ADAM BRUNET: At Dwelltime Café in Cambridge, Massachusetts, one of the most popular solutions on the planet is being prepared daily. ETHAN MILLER: I became really interested in coffee when I started working at a coffee shop just as a summer job and sort of fell in love with all the intricacies of how it works and how you can change it with really little changes in parameters when you're brewing coffee. When you roast coffee, the taste changes. When it s very lightly roasted, it s very grassy and a little bit sour. As you roast it a little bit more and more, it becomes a little bit sweeter and a little bit more fruity. And then the darker you get, the more you go into the smoky and bitter. Before you roast coffee, it's green and hard. It doesn't smell like much. And it's not really soluble. So you need to roast it in order to get out the 900 to 3,000 different solutes. You know, roasted coffee is actually a composite of thousands of different solutes. When we roast green coffee, we bring it up to about 210 Celsius or maybe around 330 Fahrenheit. TIMOTHY BORREGO: You re basically cooking this green product and it s going to change it. So in the rotating drum is the green coffee, which is almost not soluble at all. It s going to taste like a terrible cup of coffee if you try to brew it. And in the cooling tray here, you have the roasted coffee, which is going to taste really delicious and aromatic and flavorful something that you are going to wake up with and get you moving on your day.
3 Unit 8: When Chemicals Meet Water The Properties of Solutions 3 ETHAN MILLER: So when we're going to make a cup of coffee, we start with our water and our kettle. And we're going to slowly pour that over our ground coffee. And what's going to happen then is our really hot solvent, is going to combine with our solute, our coffee. And that's going to slowly dissolve all of the 900 to 3,000 different chemical compounds inside of it that give us our brewed coffee. But it's kind of a strange way to think of coffee as dissolving, because you're left with all of these grounds afterwards, and really all you re getting rid of is 1-2% of your coffee. When people try cold-brew for the first time, a lot of them are really surprised. It doesn't have some of the sourness that a hot coffee might have. It's usually really strong and very chocolaty because of the way we brew it. TIM BORREGO: I ve got about 1000 milliliters of water and 100 grams of coffee, a one to ten ratio. This coarse grind is less soluble compared to a really fine grind. We re using cold water to start off with and we re going to put it in the refrigerator for 24 hours. This is going to differ quite a bit from hot brew in that you can control the extraction versus if you had a hot brew, you re going to extract things very quickly with that high temperature and at lower temperatures over time, you can extract a lot of the really great stuff before you ever reach the point where you re starting to extract some of the more bitter aspects of coffee. It s got everything in there that you d want from, you know, a good cup of cold coffee. ETHAN MILLER: So we have the hot brew coffee, and the cold brew coffee. In the battle of solubility, who will win? And now we re going to check the TDS of each one, which is the total dissolved solids. We re going to find out what percentage of our solution is made up of solutes. We re going to use a TDS meter and its job is to use an electrical current to tell us how many dissolved solids are in each cup of coffee. We re going to start with the hot brew coffee. Now I m going to jump right into weighing out the cold brew. I m going to start by checking the total dissolved solids of the hot brew. And right now we re getting a reading of 735, 736 parts per million. 736 parts per million means that we have a fairly high percentage of our solution is made up of solutes. Now we re going to go into our cold brewed coffee and check the parts per million of solutes in that guy. Looks like this guy has only got 624 parts per million. So it s a much lower percentage of solutes in our solution. Did temperature play a difference in solubility? Absolutely. The hot brew coffee ended up being way more soluble than the cold brew. Espresso is a really strong rich cup of coffee that s made with a machine that forces hot water through coffee. The variable that we re going to change when we do an espresso is we re adding a whole lot of pressure to the mix.
4 Unit 8: When Chemicals Meet Water The Properties of Solutions 4 So this is a portafilter. This is where the coffee goes when we want to make an espresso. And this guy right here is where all the magic happens. Ground the coffee nice and fine so we have lots of surface area to work with. It s going to make our coffee really soluble. Now we re going to put the espresso in the big presser. Really force it down with 130 or so psi. So let s pretend that I m the water inside an espresso machine. I m going to get really hot and I m going to be under a lot of pressure. So when I hit that coffee, I m going to make things a lot more soluble than I would if we were at one atmosphere of pressure. In that cup, we re going to have this wonderful colloid, which is going to be a suspension of fats and non-dissolved particles as well, so it s going to be really different than just a brewed cup of coffee. So now we have our finished espresso. As you can see it has this brown, creamy fluid on top, and you can see the solution poking through underneath. And that s an emulsification of fats that have come through, because we ve increased the pressure a lot and that s made the fats way more soluble and that s how they ended up in our cup. At nine atmospheres of pressure the fats are really soluble, and now that we have it in this cup at one atmosphere of pressure they re just not very soluble at all, and that s why they all float to the surface. This is crema, which is just the soluble fats that are inside espresso. Most people think that they re really delicious, but realistically they re just kind of bitter and gross tasting. Brewing the perfect cup of coffee isn t necessarily an art. It s not really a science either; it s kind of a seamless blend of the two, and we like to think of it more as a craft. MADISON: Here you go the perfect latte. Or close to it. [SEGMENT 3: The Greatest Solvent] ADAM BRUNET: So, why is water such a great solvent? To answer this question, we need to look at what s going on on the molecular level. I like to look at the example of salt water because it s such a simple and beautiful system. First we have the H 2 O. Now the covalent bonds between the oxygen and the hydrogen are formed by a sharing of electrons between the atomic nuclei. But this sharing is unequal: the oxygen nucleus pulls electrons to itself more strongly than the hydrogen nuclei do. This means that the electrons are a little bit more likely to be found around the oxygen, making it slightly negative, and little bit less likely to be found around the hydrogens, making them slightly positive. Another way of saying this is that the water molecule is a polar molecule: it has a positive end and a negative end. So now we add the salt. Table salt NaCl, sodium chloride is an ionic compound, made up of a positively charged sodium ion and a negatively charged chloride ion. As
5 Unit 8: When Chemicals Meet Water The Properties of Solutions 5 charged objects are attracted to other charged objects of opposite sign, a polar molecule like water can interact strongly with these ions to help them dissolve. Let s look at how this works in more detail. When the salt comes in contact with water, the positive sodium ions can become surrounded by the negative oxygen ends of the water molecules. And the negatively charged chloride ions become surrounded by the positive ends of the water molecules. And the separate sodium and chloride ions can then diffuse throughout the entire volume of water, distributing themselves evenly. When all of the molecules are dispersed in solution, it is the perfect environment for a beautiful reaction to take place. [SEGMENT 4: DEMO Solids in Solution] DANIEL ROSENBERG: Let s see what happens when I try to get two solids to react by mixing. One of them is lead nitrate. The other one is potassium iodide. And when they react, they form a yellow compound. So let s see what happens when I just mix up the dry powders. There s not much happening there, but why isn t there much happening there? It s like the two big grains of lead nitrate and potassium iodide are bumping against each other, but there s a lot inside and there s not much on the outside, there s not a whole lot of surface area that can react. And so we have to figure out a way that we can give these two solids more surface area to react. What s the solution? We make a solution. So I m adding water to the potassium iodide, and as I stir it, these large grains of salt are dissolving in the water. So they re going from a dry chemical to a solution where the potassium iodide is evenly distributed through the entire solution. Now we can make the lead nitrate solution. We re taking a dry powder, and we re dissolving it in water to make a solution of lead nitrate. So, we ve gone from a place where we had big pieces of material that could only react on their surfaces to the lead nitrate evenly distributed through the solution, ready to react with every part of the potassium iodide in this solution. So let s see what happens when we mix them together. But I m going to do it a little bit at a time. Each time I add, I get a burst of color. Those solutions can react as fast as they can mix. So now, I m going to pour in the rest, and finish that reaction up. So look how quickly that happened. As quickly as we mixed those two solutions, we got lead iodide precipitating out of this solution, and it s a beautiful yellow color. In a solution, the molecules are free to move around and interact with each other without regard to surface area. They bump up against each other and they react. And that s why solutions are so powerful in chemistry. The potassium iodide, KI, and lead nitrate, Pb(NO 3 ) 2, like table salt, are compounds
6 Unit 8: When Chemicals Meet Water The Properties of Solutions 6 with negatively and positively charged ions that can dissolve in water. Once dissolved, they can recombine in what s called a double displacement reaction. Lead ions and iodide ions combine to make lead iodide, an insoluble compound that precipitates out of solution as a solid. The potassium cations and the nitrate anions are left behind still dissolved in the solution. This potassium nitrate that remains is a soluble ionic compound, and the positive and negative ions are stabilized in the solution by the polarity of the water. [SEGMENT 4: Gases in Solution] ADAM BRUNET: Most of the solutions we ve looked at so far were solids dissolved in an aqueous solution. Aqueous just means that the solvent was water. But I m also very interested in another group of solutions, solutions where the solute is a gas. Understanding that gases can dissolve in water is critical to my ability to dive and return to the surface alive. [SEGMENT 5: DEMO The Ammonia Fountain] DANIEL ROSENBERG: This is the ammonia fountain. This is a fun way of showing that gas can dissolve in water. In this flask, I have one thousand milliliters of ammonia gas at atmospheric pressure. In order to make this reaction take place, I m going to add 2mL of water with an indicator that will show us when water and ammonia have mixed to form ammonia water. Now the ammonia fountain reaction happens the second that I inject this water into this flask. The ammonia gas is going to dissolve into that water entirely, leaving 2mL of ammonia solution, and nothing else in the flask at top. We ll know it s happening because the water will turn pink. So in the top flask, all of the ammonia has dissolved into this water, and we can see that because the indicator is showing a basic solution. All of this gas in that tiny amount of solution means that up here is a partial vacuum. A partial vacuum connected with a flask full of water and I can open up this stopcock so that the partial vacuum at the top can communicate with the atmospheric pressure water at the bottom. The difference in pressure forces the liquid up into the flask at top. And that is the ammonia fountain. So what s interesting is that a gas can dissolve in a liquid. ADAM BRUNET: Understanding how gases dissolve in water is critical to Scuba divers who must be very conscious of decompression sickness, commonly called the bends. The bends is a nitrogen gas solubility problem. The air we breathe is 78% nitrogen, and nitrogen dissolves readily from the lungs into a solution called blood, which is mostly water, and flows through all the tissues of our body.
7 Unit 8: When Chemicals Meet Water The Properties of Solutions 7 The amount of nitrogen in your system is affected by the pressure of the air you breathe. As you dive deeper into the ocean, the pressure increases. When the pressure increases the nitrogen becomes more soluble, so there is more nitrogen dissolved in your blood, a relationship described by Henry's Law. Henry's Law states that the solubility of a gas in a liquid is proportional to the partial pressure of the gas over the solution. We say partial pressure because the air a diver breathes from his tank, is a solution of multiple gases and the nitrogen is only a part of the air, so we are talking about the partial pressure due to nitrogen. So this all becomes an issue that can affect the divers life, because as he moves from the deep water back to the surface, the pressure decreases dramatically and the nitrogen that was soluble in the blood at depth is no longer soluble at the lower surface pressure. The nitrogen then comes out of solution before it reaches the lungs, creating many gas bubbles in the blood that are very painful and can lead to death. I was swimming around at a relatively shallow depth. The difference in pressure between being about down about 20 feet and being at the surface isn t that much. But as I go deeper, the air I m breathing is subject to greater and greater pressure from the weight of all that water. And the amount of nitrogen in my blood can reach dangerous levels. In order to avoid the bends, I need to take my time coming back to the surface, so that the nitrogen in my blood can escape slowly as I breathe, and my body can adapt to the changing environment. Understanding how gas is dissolved in a diver s blood is important, but gases don t just dissolve in liquids in a biological solution like blood. They re also important here, where the atmosphere meets the ocean. [SEGMENT 7: CO 2 in the Water] At the Smithsonian Environmental Research Center, Whitman Miller and his group are studying the effects of increased atmospheric carbon dioxide on the oceans. FRITZ REIDEL: Around us we have the atmosphere. It s a solution of gases, composed primarily of nitrogen and oxygen, but also has a large number of trace gases of which carbon dioxide is one of the most important for life and is also a potential hazard and toxin. WHITMAN MILLER: So many people have heard of global warming through the greenhouse effect, and CO 2 is one of the greenhouse gases that produces that effect.
8 Unit 8: When Chemicals Meet Water The Properties of Solutions 8 However, what people don't necessarily know is all of that CO 2 that goes up into the atmosphere doesn't stay there. About a quarter of it actually goes into the world's oceans. And so, the gas solution from the atmosphere actually mixes with the open ocean. And as it does that, it changes the chemistry of the ocean. FRITZ REIDEL: Simply put, the higher the concentrations of carbon dioxide in the atmosphere, the higher the concentration of carbon dioxide is going to be in the oceans. WHITMAN MILLER: And since CO 2 functions as an acid in the ocean, the oceans are becoming more acidic. Miller s team is concerned that the increasing acidity that comes with increased CO 2 levels in the water will have devastating effects on the marine life and ecosystem in the future. Their concern stems from earlier research on the growth of oyster larvae. WHITMAN MILLER: We did some laboratory-based experiments where we wanted to understand how changing CO 2 concentrations in the water that oyster larvae were grown in, how that affected their growth and how it affected how they make their shells, or what we call the calcification of their shells. The team found a significant difference between the growth of oysters in preindustrial CO 2 conditions of 280ppm and in elevated CO 2 conditions of 800ppm, which is what is predicted for 100 years in the future. WHITMAN MILLER: So what we found was that under high CO 2 conditions, the oysters grew significantly slower, and that they calcified less. So, since we ve done the laboratory experiments and knew those were somewhat simplified, we want to know what s happening in the natural environment. And so, the first step to do that is to understand what the natural baseline dynamics of pco 2 and ph are, in say, the Chesapeake Bay. But as it turns out, the Chesapeake Bay is a very complex place, and this part of the Chesapeake Bay is also very complicated. And what that means is that we need to sample intensively across time, but also across space, because what is happening here, in this location may be quite different than what s happening just a kilometer away. While in the open ocean CO 2 levels are fairly consistent, in costal systems where these oysters live the proximity to the land creates wildly fluctuating CO 2 levels. WHITMAN MILLER: In coastal systems there s an interaction between the land and the sea. And in this location, we're at an estuarine salt marsh. And so, this salt marsh is actually, it turns out, exporting a lot of CO 2. And that CO 2 actually comes from the growth of these plants through photosynthesis, which is harvesting CO 2 out of the air. When that
9 Unit 8: When Chemicals Meet Water The Properties of Solutions 9 plant dies, it goes to the ground and begins to decay. And as this decays, the CO 2 is released. And that CO 2 makes its way into these tidal creeks. And so we want to understand the dynamics in these coastal systems because they're much more complicated than what's going on in the open ocean. And we need to understand what those dynamics are like with respect to night and day, with respect to tidal cycles, with respect to closeness to a salt marsh or an upland forest. The team sets out to measure the CO 2 levels in the water throughout the day and across a variety of locations. To measure the amount of gas in a liquid, they use a special device, called an equilibrator. WHITMAN MILLER: So this device is called an equilibrator. Its purpose is to help measure the concentration of CO 2 in that water. So currently, the air in these tubes is at atmosphere. There's a vent straight to the atmosphere. However, when I turn on this valve, it begins pumping water from this creek in. So what happens, as this water comes into these pipes, it mixes with the air space in the equilibrator and, through Henry's Law, the water and the air come into equilibrium. So therefore, if we take a sample of the air, it's reflective of the concentration of CO 2 in the water. Throughout the Chesapeake Bay, the team has recorded levels of CO 2 varying dramatically from 100ppm to 15,000ppm. Once they understand the baseline dynamics for CO 2 in this area, they can begin experiments to predict how oysters will fare in these conditions in the future, when CO 2 levels are predicted to be even higher. AMANDA REYNOLDS: So we are going to take juvenile oysters, and we are going to put them in the river so they experience their natural environment. And we re actually going to be adding in extra CO 2, using these bubblers that will be bubbling in CO 2. And so, we re going to take say, the changing CO 2 in the water, what already occurs naturally, which might look like this up and down, up and down. And we re going to actually elevate that whole graph up a level. So instead of being down here, we re going to elevate it so the change is kicked up a notch. And that way, we can try to predict what might happen to oysters one hundred years from now, when the levels of CO 2 will potentially effect our oyster populations in the Chesapeake Bay. WHITMAN MILLER: One goal of ours is to try to understand whether CO 2 and ph are important in locating restoration sites, however, understanding the dynamics now and then projecting what may happen in a high CO 2 world may also inform us about where we do restoration efforts in the future. [WRAP-UP] ADAM BRUNET: Solutions are all around us. There are gaseous solutions, like the air, aqueous solutions, like the ocean or biological systems, like my blood and there s solid solutions, like the steel alloy. Understanding how they work is critical in understanding
10 Unit 8: When Chemicals Meet Water The Properties of Solutions 10 the impact that they have on our lives every day. [END]
Solubility Lab Packet
 Solubility Lab Packet **This packet was created using information gathered from the American Chemical Society s Investigation #4: Dissolving Solids, Liquids, and Gases (2007). It is intended to be used
Solubility Lab Packet **This packet was created using information gathered from the American Chemical Society s Investigation #4: Dissolving Solids, Liquids, and Gases (2007). It is intended to be used
Properties of Water Lab: What Makes Water Special? An Investigation of the Liquid That Makes All Life Possible: Water!
 Properties of Water Lab: What Makes Water Special? An Investigation of the Liquid That Makes All Life Possible: Water! Background: Water has some peculiar properties, but because it is the most common
Properties of Water Lab: What Makes Water Special? An Investigation of the Liquid That Makes All Life Possible: Water! Background: Water has some peculiar properties, but because it is the most common
The grade 5 English science unit, Solutions, meets the academic content standards set in the Korean curriculum, which state students should:
 This unit deals with how solids dissolve in liquids and what affects their dissolution. By studying the dissolution process and related factors, students develop an interest in and curiosity about solutions.
This unit deals with how solids dissolve in liquids and what affects their dissolution. By studying the dissolution process and related factors, students develop an interest in and curiosity about solutions.
The Science of Lemonade
 Design your own recipe for lemonade using lemons, sugar, and water. On the basis of what you learned, decide how many lemons and how much water and sugar to use. Make your lemonade and then taste it. Is
Design your own recipe for lemonade using lemons, sugar, and water. On the basis of what you learned, decide how many lemons and how much water and sugar to use. Make your lemonade and then taste it. Is
Molecular Gastronomy: The Chemistry of Cooking
 Molecular Gastronomy: The Chemistry of Cooking We re surrounded by chemistry each and every day but some instances are more obvious than others. Most people recognize that their medicine is the product
Molecular Gastronomy: The Chemistry of Cooking We re surrounded by chemistry each and every day but some instances are more obvious than others. Most people recognize that their medicine is the product
Distillation Purification of Liquids
 Distillation Purification of Liquids Types of Distillations commonly used in Organic Lab: Simple separates volatile compounds (liquids) from non-volatile compounds (solids) or volatiles with boiling points
Distillation Purification of Liquids Types of Distillations commonly used in Organic Lab: Simple separates volatile compounds (liquids) from non-volatile compounds (solids) or volatiles with boiling points
TACO SAUCE PENNY CLEANER
 TACO SAUCE PENNY CLEANER Next: Materials and Explanations www.stevespanglerscience.com Then: Step-by-Step Photo Sequence TACO SAUCE PENNY CLEANER It's one of those things you hear about but wonder if it's
TACO SAUCE PENNY CLEANER Next: Materials and Explanations www.stevespanglerscience.com Then: Step-by-Step Photo Sequence TACO SAUCE PENNY CLEANER It's one of those things you hear about but wonder if it's
Separations. Objective. Background. Date Lab Time Name
 Objective Separations Techniques of separating mixtures will be illustrated using chromatographic methods. The natural pigments found in spinach leaves, β-carotene and chlorophyll, will be separated using
Objective Separations Techniques of separating mixtures will be illustrated using chromatographic methods. The natural pigments found in spinach leaves, β-carotene and chlorophyll, will be separated using
Notes on acid adjustments:
 Notes on acid adjustments: In general, acidity levels in 2018 were lower than normal. Grape acidity is critical for the winemaking process, as well as the quality of the wine. There are 2 common ways to
Notes on acid adjustments: In general, acidity levels in 2018 were lower than normal. Grape acidity is critical for the winemaking process, as well as the quality of the wine. There are 2 common ways to
COFFEE BASICS SCAA. The Elements of Proper Brewing and Creating an Ideal Coffee Drinking Experience
 COFFEE BASICS The Elements of Proper Brewing and Creating an Ideal Coffee Drinking Experience SCAA WATER THE ELEMENTS OF PROPER BREWING Fresh, good-tasting water is essential since it makes up more than
COFFEE BASICS The Elements of Proper Brewing and Creating an Ideal Coffee Drinking Experience SCAA WATER THE ELEMENTS OF PROPER BREWING Fresh, good-tasting water is essential since it makes up more than
CHEM Experiment 4 Introduction to Separation Techniques I. Objectives
 1 CHEM 0011 Experiment 4 Introduction to Separation Techniques I Objectives 1. To learn the gravity filtration technique 2. To learn the suction filtration technique 3. To learn about solvent extraction
1 CHEM 0011 Experiment 4 Introduction to Separation Techniques I Objectives 1. To learn the gravity filtration technique 2. To learn the suction filtration technique 3. To learn about solvent extraction
Station 1. Polarity of Water
 Station 1 Polarity of Water As we learned last week, water is a polar molecule meaning it has one end with a slight positive charge and another end with a slight negative charge. Molecules without slight
Station 1 Polarity of Water As we learned last week, water is a polar molecule meaning it has one end with a slight positive charge and another end with a slight negative charge. Molecules without slight
Lab 2. Drug Abuse. Solubility and Colligative Properties of Solutions: Coffee, Soda, and Ice Cream
 Lab 2. Drug Abuse. Solubility and Colligative Properties of Solutions: Coffee, Soda, and Ice Cream How do I make a stronger cup of coffee? How do I make ice cream? Prelab Spend 5 minutes doing the following
Lab 2. Drug Abuse. Solubility and Colligative Properties of Solutions: Coffee, Soda, and Ice Cream How do I make a stronger cup of coffee? How do I make ice cream? Prelab Spend 5 minutes doing the following
THE EGG-CITING EGG-SPERIMENT!
 1 of 5 11/1/2011 10:30 AM THE EGG-CITING EGG-SPERIMENT! Knight Foundation Summer Institute Arthurea Smith, Strawberry Mansion Middle School Liane D'Alessandro, Haverford College Introduction: Get ready
1 of 5 11/1/2011 10:30 AM THE EGG-CITING EGG-SPERIMENT! Knight Foundation Summer Institute Arthurea Smith, Strawberry Mansion Middle School Liane D'Alessandro, Haverford College Introduction: Get ready
The Separation of a Mixture into Pure Substances
 The Separation of a Mixture into Pure Substances The experiment is designed to familiarize you with some standard chemical techniques and to encourage careful work in separating and weighing chemicals.
The Separation of a Mixture into Pure Substances The experiment is designed to familiarize you with some standard chemical techniques and to encourage careful work in separating and weighing chemicals.
Adapted By Kennda Lynch, Elizabeth Adsit and Kathy Zook July 26, Moooooogic!
 Moooooogic! Objective: Students will use the scientific method to test the difference between using whole milk and skim milk in this milk and food dye experiment. Students will explore ideas of density,
Moooooogic! Objective: Students will use the scientific method to test the difference between using whole milk and skim milk in this milk and food dye experiment. Students will explore ideas of density,
COFFEE BASICS. The Elements of Proper Brewing and Creating an Ideal Coffee Drinking Experience SCAA
 COFFEE BASICS The Elements of Proper Brewing and Creating an Ideal Coffee Drinking Experience SCAA WATER THE ELEMENTS OF Proper BREWING Fresh, good-tasting water is essential since it makes up more than
COFFEE BASICS The Elements of Proper Brewing and Creating an Ideal Coffee Drinking Experience SCAA WATER THE ELEMENTS OF Proper BREWING Fresh, good-tasting water is essential since it makes up more than
Mix the Old with the New
 Mix the Old with the New Chefs in busy restaurants do a lot of different things. They check the inventory of ingredients used for each popular dish. They may supervise a kitchen staff, making sure their
Mix the Old with the New Chefs in busy restaurants do a lot of different things. They check the inventory of ingredients used for each popular dish. They may supervise a kitchen staff, making sure their
Experiment 3: Separation of a Mixture Pre-lab Exercise
 1 Experiment 3: Separation of a Mixture Pre-lab Exercise Name: The amounts of sand, salt, and benzoic acid that will dissolve in 100 g of water at different temperatures: Temperature 0 C 20 C 40 C 60 C
1 Experiment 3: Separation of a Mixture Pre-lab Exercise Name: The amounts of sand, salt, and benzoic acid that will dissolve in 100 g of water at different temperatures: Temperature 0 C 20 C 40 C 60 C
The Lazy Mans Guide to Extracting Mimosa Hostilis Root Bark by Vortex A report and guide for a new way of extracting MHRB
 The Lazy Mans Guide to Extracting Mimosa Hostilis Root Bark by Vortex A report and guide for a new way of extracting MHRB Extraction Time: 1 gm in 2.5 hrs, 4 gm in 7 hr, 7.5 g total @48 hrs Equipment:
The Lazy Mans Guide to Extracting Mimosa Hostilis Root Bark by Vortex A report and guide for a new way of extracting MHRB Extraction Time: 1 gm in 2.5 hrs, 4 gm in 7 hr, 7.5 g total @48 hrs Equipment:
The Floating Leaf Disk Assay for Investigating Photosynthesis
 The Floating Leaf Disk Assay for Investigating Photosynthesis The biology behind the procedure: Leaf disks float, normally. When the air spaces are infiltrated with solution the overall density of the
The Floating Leaf Disk Assay for Investigating Photosynthesis The biology behind the procedure: Leaf disks float, normally. When the air spaces are infiltrated with solution the overall density of the
1. What is made when a solute is dissolved in a solvent?
 A solution is made when a solute dissolves in a solvent. The solutions we will look at are those where a solid dissolves in a liquid. The solid is the solute and the liquid is the solvent. Solute + Solvent
A solution is made when a solute dissolves in a solvent. The solutions we will look at are those where a solid dissolves in a liquid. The solid is the solute and the liquid is the solvent. Solute + Solvent
Lab 2. Drug Abuse. Solubility and Colligative Properties of Solutions: Coffee, Soda, and Ice Cream
 Lab 2. Drug Abuse. Solubility and Colligative Properties of Solutions: Coffee, Soda, and Ice Cream How do I make a stronger cup of coffee? How do I make ice cream? Prelab Spend 5 minutes doing the following
Lab 2. Drug Abuse. Solubility and Colligative Properties of Solutions: Coffee, Soda, and Ice Cream How do I make a stronger cup of coffee? How do I make ice cream? Prelab Spend 5 minutes doing the following
Colour Changing Christmas Tree
 Colour Changing Christmas Tree Create a Christmas tree centrepiece that changes from plain white to red and green before your eyes, using pantry ingredients for some kitchen chemistry. You ll need A few
Colour Changing Christmas Tree Create a Christmas tree centrepiece that changes from plain white to red and green before your eyes, using pantry ingredients for some kitchen chemistry. You ll need A few
7.2.4 Mixtures. 100 minutes. 146 marks. Page 1 of 42
 7.2.4 Mixtures 100 minutes 146 marks Page 1 of 42 ## John ground some coffee beans into little pieces. He put them into a coffee filter and poured 800 cm 3 of boiling water over them to make a jug of coffee.
7.2.4 Mixtures 100 minutes 146 marks Page 1 of 42 ## John ground some coffee beans into little pieces. He put them into a coffee filter and poured 800 cm 3 of boiling water over them to make a jug of coffee.
2. Other constituents in the sample solution should not interfere with the precipitation of the component of interest.
 EXPERIMENT 15 Percentage Yield of Lead (II) Iodide in a Gravimetric Analysis INTRODUCTION In a gravimetric analysis, a substance is treated so that the component of interest is separated either in its
EXPERIMENT 15 Percentage Yield of Lead (II) Iodide in a Gravimetric Analysis INTRODUCTION In a gravimetric analysis, a substance is treated so that the component of interest is separated either in its
Beer Preparation for Packaging. Jamie Ramshaw M.Brew Simpsons Malt
 Beer Preparation for Packaging Jamie Ramshaw M.Brew Simpsons Malt Conditioning Cask Processed Beer Preparation Conditioning Haze and Clarity Stabilisation Conditioning Aims Flavour development Development
Beer Preparation for Packaging Jamie Ramshaw M.Brew Simpsons Malt Conditioning Cask Processed Beer Preparation Conditioning Haze and Clarity Stabilisation Conditioning Aims Flavour development Development
Mixtures and Solutions Stations Lesson Plan by Clara Welch Based on FOSS & Kitchen Chemistry by John Bath, Ph. D. and Sally Mayberry, Ed. D.
 Mixtures and Solutions Stations Lesson Plan by Clara Welch Based on FOSS & Kitchen Chemistry by John Bath, Ph. D. and Sally Mayberry, Ed. D. Overview: This lesson is a group of activities that may be used
Mixtures and Solutions Stations Lesson Plan by Clara Welch Based on FOSS & Kitchen Chemistry by John Bath, Ph. D. and Sally Mayberry, Ed. D. Overview: This lesson is a group of activities that may be used
Separating Mechanical Mixtures
 3.2 Separating Mechanical Mixtures Key Question: How can you separate mechanical mixtures? Remember from Chapter 1 that a mechanical mixture is a mixture with different parts that you can see. People work
3.2 Separating Mechanical Mixtures Key Question: How can you separate mechanical mixtures? Remember from Chapter 1 that a mechanical mixture is a mixture with different parts that you can see. People work
Cold Stability Anything But Stable! Eric Wilkes Fosters Wine Estates
 Cold Stability Anything But Stable! Fosters Wine Estates What is Cold Stability? Cold stability refers to a wine s tendency to precipitate solids when held cool. The major precipitates tend to be tartrates
Cold Stability Anything But Stable! Fosters Wine Estates What is Cold Stability? Cold stability refers to a wine s tendency to precipitate solids when held cool. The major precipitates tend to be tartrates
Assignment #3: Lava Lite!!
 Assignment #3: Lava Lite!! This activity entails making a lava lamp. PROCEDURE: GOALS: 1) Fill a glass cup with three inches of water. 2) Put about _ of an inch of oil in the water. Notice what the oil
Assignment #3: Lava Lite!! This activity entails making a lava lamp. PROCEDURE: GOALS: 1) Fill a glass cup with three inches of water. 2) Put about _ of an inch of oil in the water. Notice what the oil
mott corporation P o r o u s M e t a l P r o d u c t s High-efficiency gas/liquid contacting.
 mott corporation P o r o u s M e t a l P r o d u c t s High-efficiency gas/liquid contacting. The best media for gas/liquid contacting. Mott porous metal. There s no better media for producing miniature,
mott corporation P o r o u s M e t a l P r o d u c t s High-efficiency gas/liquid contacting. The best media for gas/liquid contacting. Mott porous metal. There s no better media for producing miniature,
Section 1.1 Classifying Matter. Classification by Composition: What is stuff made of?
 READ p.14-23 Section 1.1 Classifying Matter 7.1 Classification by Composition: What is stuff made of? Qualitative: Expresses Composition Quantitative: Uses Measurements carbon hydrogen oxygen 42% carbon
READ p.14-23 Section 1.1 Classifying Matter 7.1 Classification by Composition: What is stuff made of? Qualitative: Expresses Composition Quantitative: Uses Measurements carbon hydrogen oxygen 42% carbon
Acta Chimica and Pharmaceutica Indica
 Acta Chimica and Pharmaceutica Indica Research Vol 7 Issue 2 Oxygen Removal from the White Wine in Winery VladimirBales *, DominikFurman, Pavel Timar and Milos Sevcik 2 Faculty of Chemical and Food Technology,
Acta Chimica and Pharmaceutica Indica Research Vol 7 Issue 2 Oxygen Removal from the White Wine in Winery VladimirBales *, DominikFurman, Pavel Timar and Milos Sevcik 2 Faculty of Chemical and Food Technology,
The next experiment was with the hot acid and the balloon. What might happen this time? It might shrink.
 August 2017 National Science Week This week is National Science Week. To mark the occasion, two science teachers from the Senior School conducted some science experiments within the ELC 3 groups. Ms Harrison:
August 2017 National Science Week This week is National Science Week. To mark the occasion, two science teachers from the Senior School conducted some science experiments within the ELC 3 groups. Ms Harrison:
Mixtures. ingredients: the separate parts of a mixture
 Every day, we interact with many different kinds of matter. We look at it, feel it, taste it, and even breathe it. Sometimes different types of matter are combined. For example, a salad might have several
Every day, we interact with many different kinds of matter. We look at it, feel it, taste it, and even breathe it. Sometimes different types of matter are combined. For example, a salad might have several
Rock Candy Lab Name: D/H
 Rock Candy Lab Name: D/H What is sugar? 1 The white stuff we know as sugar is sucrose, a molecule composed of 12 atoms of carbon, 22 atoms of hydrogen, and 11 atoms of oxygen (C12H22O11). Like all compounds
Rock Candy Lab Name: D/H What is sugar? 1 The white stuff we know as sugar is sucrose, a molecule composed of 12 atoms of carbon, 22 atoms of hydrogen, and 11 atoms of oxygen (C12H22O11). Like all compounds
Tiny Bubbles. A Primer on Beer Carbonation. Chris Saunders. May 1, A Primer on Beer Carbonation 1/40
 Tiny Bubbles A Primer on Beer Carbonation Chris Saunders May 1, 2017 A Primer on Beer Carbonation 1/40 Chemistry Overview Molecular Weights, Units & Constants Ideal Gas Law Henry s Law CO 2 in Beer What
Tiny Bubbles A Primer on Beer Carbonation Chris Saunders May 1, 2017 A Primer on Beer Carbonation 1/40 Chemistry Overview Molecular Weights, Units & Constants Ideal Gas Law Henry s Law CO 2 in Beer What
Anaerobic Cell Respiration by Yeast
 25 Marks (I) Anaerobic Cell Respiration by Yeast BACKGROUND: Yeast are tiny single-celled (unicellular) fungi. The organisms in the Kingdom Fungi are not capable of making their own food. Fungi, like any
25 Marks (I) Anaerobic Cell Respiration by Yeast BACKGROUND: Yeast are tiny single-celled (unicellular) fungi. The organisms in the Kingdom Fungi are not capable of making their own food. Fungi, like any
Do heating and cooling have an effect on matter?
 Matter on the move In art class the other day, we tried making our own watercolor paint. We had food coloring and were adding drops in different combinations to water. Some kids put their drops in and
Matter on the move In art class the other day, we tried making our own watercolor paint. We had food coloring and were adding drops in different combinations to water. Some kids put their drops in and
Coffee Filter Chromatography
 Here is a summary of what you will learn in this section: Solutions can be separated by filtration, paper chromatography, evaporation, or distillation. Mechanical mixtures can be separated by sorting,
Here is a summary of what you will learn in this section: Solutions can be separated by filtration, paper chromatography, evaporation, or distillation. Mechanical mixtures can be separated by sorting,
The importance of using fresh roasted coffee
 The importance of using fresh roasted coffee Coffee tastes at its best 4-10 days after roasting, but will still taste good, if kept airtight, for up to a month. There is no problem using beans older than
The importance of using fresh roasted coffee Coffee tastes at its best 4-10 days after roasting, but will still taste good, if kept airtight, for up to a month. There is no problem using beans older than
Greenhouse Effect Investigating Global Warming
 Greenhouse Effect Investigating Global Warming OBJECTIVE Students will design three different environments, including a control group. They will identify which environment results in the greatest temperature
Greenhouse Effect Investigating Global Warming OBJECTIVE Students will design three different environments, including a control group. They will identify which environment results in the greatest temperature
A naked egg is an egg without a shell. Using vinegar, you can dissolve the eggshell without breaking the membrane that contains the egg.
 A naked egg is an egg without a shell. Using vinegar, you can dissolve the eggshell without breaking the membrane that contains the egg. What Do I Need?. a few eggs white vinegar a container big enough
A naked egg is an egg without a shell. Using vinegar, you can dissolve the eggshell without breaking the membrane that contains the egg. What Do I Need?. a few eggs white vinegar a container big enough
EXTRACTION. Extraction is a very common laboratory procedure used when isolating or purifying a product.
 EXTRACTION Extraction is a very common laboratory procedure used when isolating or purifying a product. Extraction is the drawing or pulling out of something from something else. By far the most universal
EXTRACTION Extraction is a very common laboratory procedure used when isolating or purifying a product. Extraction is the drawing or pulling out of something from something else. By far the most universal
3rd Grade Changes Assessment
 Name Date 1. Yong bought a can of soda at the pool and left the soda in the Sun while he swam. When Yong came back, the soda can was warm. What raised the temperature of his soda? A. the sound of people's
Name Date 1. Yong bought a can of soda at the pool and left the soda in the Sun while he swam. When Yong came back, the soda can was warm. What raised the temperature of his soda? A. the sound of people's
strada ep guidebook an introduction to pressure profiling, and a guide to the la marzocco strada ep rev. 1.1
 strada ep guidebook an introduction to pressure profiling, and a guide to the la marzocco strada ep rev. 1.1 Via La Torre 14/H 50038 Scarperia Florence - ITALY phone: +39 055 849 191 fax: +39 055 849 1990
strada ep guidebook an introduction to pressure profiling, and a guide to the la marzocco strada ep rev. 1.1 Via La Torre 14/H 50038 Scarperia Florence - ITALY phone: +39 055 849 191 fax: +39 055 849 1990
Breathless Balloon. Tools:
 Breathless Balloon Tools: Extras: Baking soda; 12" Round balloon; Vinegar Key Science Concept: Acids and bases react to make a gas called carbon dioxide. Caution: Perform this experiment only under adult
Breathless Balloon Tools: Extras: Baking soda; 12" Round balloon; Vinegar Key Science Concept: Acids and bases react to make a gas called carbon dioxide. Caution: Perform this experiment only under adult
Sample Questions for the Chemistry of Coffee Topic Test
 Sample Questions for the Chemistry of Coffee Topic Test 1. During the 2013 Barista Championship, one of the contestants used a distillation apparatus to deliver a distilled coffee product as his specialty
Sample Questions for the Chemistry of Coffee Topic Test 1. During the 2013 Barista Championship, one of the contestants used a distillation apparatus to deliver a distilled coffee product as his specialty
BEHAVIOR OF HOT AND COLD
 City Academy Science Kitchen Chemistry Winter STEAM Packet NAME: _ INTRODUCTION: In both science and STEAM class, students were introduced to the chemical and physical properties of matter during their
City Academy Science Kitchen Chemistry Winter STEAM Packet NAME: _ INTRODUCTION: In both science and STEAM class, students were introduced to the chemical and physical properties of matter during their
Jane Student - 6B. Nose Creek School. April 29, 2016
 1 Does Flavor Affect the Melting Process of Ice Cream? Jane Student - 6B Nose Creek School April 29, 2016 2 Purpose The purpose of this project was to find out what flavored ice cream melts the fastest.
1 Does Flavor Affect the Melting Process of Ice Cream? Jane Student - 6B Nose Creek School April 29, 2016 2 Purpose The purpose of this project was to find out what flavored ice cream melts the fastest.
Particle model of solids, liquids and gases/ solutions
 Medway LEA Advisory Service Particle model of solids, liquids and gases/ solutions 7G & 7H 32 min 32 marks Q1-L3, Q2-L4, Q3-L4, Q4-L5, Q5-L5, Q6-L6 1. Some pupils carried out an investigation to find out
Medway LEA Advisory Service Particle model of solids, liquids and gases/ solutions 7G & 7H 32 min 32 marks Q1-L3, Q2-L4, Q3-L4, Q4-L5, Q5-L5, Q6-L6 1. Some pupils carried out an investigation to find out
INTRODUCTION TO CUSTOM FABRICATED STRAINERS
 INTRODUCTION TO CUSTOM FABRICATED STRAINERS Nothing Too Big, Too Small or Too Special When unwanted solid material has to be removed from flowing fluids in order to protect equipment, a HAYWARD Strainer
INTRODUCTION TO CUSTOM FABRICATED STRAINERS Nothing Too Big, Too Small or Too Special When unwanted solid material has to be removed from flowing fluids in order to protect equipment, a HAYWARD Strainer
The Truth About Cast Iron Pans: 7 Myths That Need To Go Away
 The Truth About Cast Iron Pans: 7 Myths That Need To Go Away Myth #1: Cast iron is difficult to maintain. The Theory: Cast iron is a material that can rust, chip, or crack easily. Buying a cast iron skillet
The Truth About Cast Iron Pans: 7 Myths That Need To Go Away Myth #1: Cast iron is difficult to maintain. The Theory: Cast iron is a material that can rust, chip, or crack easily. Buying a cast iron skillet
Mentos very quickly and in huge quantities and shoot out of the bottle.
 RESEARCH Mint Mentos have lots of tiny craters inside them that create greater surface area. Having more surface area causes more reaction. The Mentos sink to the bottom of the bottle. Carbon dioxide bubbles
RESEARCH Mint Mentos have lots of tiny craters inside them that create greater surface area. Having more surface area causes more reaction. The Mentos sink to the bottom of the bottle. Carbon dioxide bubbles
Moving Molecules The Kinetic Molecular Theory of Heat
 Moving Molecules The Kinetic Molecular Theory of Heat Purpose: The purpose of this lab is for students to determine the relationship between temperature and speed of molecules in a liquid. Key Science
Moving Molecules The Kinetic Molecular Theory of Heat Purpose: The purpose of this lab is for students to determine the relationship between temperature and speed of molecules in a liquid. Key Science
HONEY. Food and Agriculture Organization of the United Nations
 HONEY Food and Agriculture Organization of the United Nations HONEY 1.- Honey General Information Honey has a fluid, crystallized (total or partially) consistence. Present a high viscosity and density
HONEY Food and Agriculture Organization of the United Nations HONEY 1.- Honey General Information Honey has a fluid, crystallized (total or partially) consistence. Present a high viscosity and density
LN 2 Ice Cream. Leading Question: Why do we stir ice cream while we freeze it?
 LN 2 Ice Cream Leading Question: Why do we stir ice cream while we freeze it? Chemical Principles: States of matter, temperature, temperature scales (Celsius, Kelvin, & Fahrenheit), solutions/mixtures,
LN 2 Ice Cream Leading Question: Why do we stir ice cream while we freeze it? Chemical Principles: States of matter, temperature, temperature scales (Celsius, Kelvin, & Fahrenheit), solutions/mixtures,
Brewing Water Derek Colby
 Brewing Water Derek Colby Minerals and Brewing Chemistry Ionic content comes from soil and rocks in its environment Ionic content of brewing water affects mashing performance and flavor perceptions in
Brewing Water Derek Colby Minerals and Brewing Chemistry Ionic content comes from soil and rocks in its environment Ionic content of brewing water affects mashing performance and flavor perceptions in
Experiment 6 Thin-Layer Chromatography (TLC)
 Experiment 6 Thin-Layer Chromatography (TLC) OUTCOMES After completing this experiment, the student should be able to: explain basic principles of chromatography in general. describe important aspects
Experiment 6 Thin-Layer Chromatography (TLC) OUTCOMES After completing this experiment, the student should be able to: explain basic principles of chromatography in general. describe important aspects
water measuring cup zipper-lock plastic sandwich bags paper towel tablespoon baking soda vinegar
 Page 1 of 5 water measuring cup zipper-lock plastic sandwich bags paper towel tablespoon baking soda vinegar Figure out where you want to explode your Bubble Bomb. Sometimes the bags make a mess when they
Page 1 of 5 water measuring cup zipper-lock plastic sandwich bags paper towel tablespoon baking soda vinegar Figure out where you want to explode your Bubble Bomb. Sometimes the bags make a mess when they
MULTIVAC BETTER PACKAGING. Multivac Southern Africa
 MULTIVAC BETTER PACKAGING Multivac Southern Africa Where do we come from? MULTIVAC Wolfertschwenden, South of Munich, current size approx. 30 000 square meters and expanding, and employing some 1500 people.
MULTIVAC BETTER PACKAGING Multivac Southern Africa Where do we come from? MULTIVAC Wolfertschwenden, South of Munich, current size approx. 30 000 square meters and expanding, and employing some 1500 people.
FERMENTATION. By Jeff Louella
 FERMENTATION By Jeff Louella Why Understand Fermentation? Understanding the science behind fermentation can greatly affect the quality of beer made. There are some great products on the market to help
FERMENTATION By Jeff Louella Why Understand Fermentation? Understanding the science behind fermentation can greatly affect the quality of beer made. There are some great products on the market to help
Bread. Guided Inquiry Activity #27
 Bread Model 1: Wheat flour is ~70-80% starch and 7-15% protein. Surprisingly, it is that relatively small percentage of protein that makes it possible for wheat flour to turn into bread. Differences in
Bread Model 1: Wheat flour is ~70-80% starch and 7-15% protein. Surprisingly, it is that relatively small percentage of protein that makes it possible for wheat flour to turn into bread. Differences in
A Salty Solution " " Consider This! Why do road crews put salt on roads in the winter to keep them safe?
 A Salty Solution Consider This! Why do road crews put salt on roads in the winter to keep them safe? The answer to the above question can be answered by studying how ice cream is made. How great is that?
A Salty Solution Consider This! Why do road crews put salt on roads in the winter to keep them safe? The answer to the above question can be answered by studying how ice cream is made. How great is that?
Preparation 1: Chloroform
 SECTION 3: General Lab Procedures Part 3: The Preparation of General Lab Chemicals General laboratory processes involve those chemical reactions where basic chemicals are being reacted, and produced. General
SECTION 3: General Lab Procedures Part 3: The Preparation of General Lab Chemicals General laboratory processes involve those chemical reactions where basic chemicals are being reacted, and produced. General
How to fine-tune your wine
 How to fine-tune your wine Fining agents help remove undesirable elements or compounds to improve the quality of wine. Fining is not just used in wines for bottle preparation, in some cases there are more
How to fine-tune your wine Fining agents help remove undesirable elements or compounds to improve the quality of wine. Fining is not just used in wines for bottle preparation, in some cases there are more
Cambridge International Examinations Cambridge International General Certificate of Secondary Education
 Cambridge International Examinations Cambridge International General Certificate of Secondary Education *8122929106* ENVIRONMENTAL MANAGEMENT 0680/11 Paper 1 October/November 2015 1 hour 30 minutes Candidates
Cambridge International Examinations Cambridge International General Certificate of Secondary Education *8122929106* ENVIRONMENTAL MANAGEMENT 0680/11 Paper 1 October/November 2015 1 hour 30 minutes Candidates
Wine-Tasting by Numbers: Using Binary Logistic Regression to Reveal the Preferences of Experts
 Wine-Tasting by Numbers: Using Binary Logistic Regression to Reveal the Preferences of Experts When you need to understand situations that seem to defy data analysis, you may be able to use techniques
Wine-Tasting by Numbers: Using Binary Logistic Regression to Reveal the Preferences of Experts When you need to understand situations that seem to defy data analysis, you may be able to use techniques
Comparative determination of glycosides in senna by using different methods of extraction (Soxhlet, maceration and ultrasonic bath)
 1 Experiment 1, 2 and 3 Comparative determination of glycosides in senna by using different methods of extraction (Soxhlet, maceration and ultrasonic bath) Aim: determine the yield among different extraction
1 Experiment 1, 2 and 3 Comparative determination of glycosides in senna by using different methods of extraction (Soxhlet, maceration and ultrasonic bath) Aim: determine the yield among different extraction
Alcoholic Fermentation in Yeast A Bioengineering Design Challenge 1
 Alcoholic Fermentation in Yeast A Bioengineering Design Challenge 1 I. Introduction Yeasts are single cell fungi. People use yeast to make bread, wine and beer. For your experiment, you will use the little
Alcoholic Fermentation in Yeast A Bioengineering Design Challenge 1 I. Introduction Yeasts are single cell fungi. People use yeast to make bread, wine and beer. For your experiment, you will use the little
Certified Home Brewer Program. Minimum Certification Requirements
 Certified Home Brewer Program Minimum Certification Requirements SCA's Minimum Certification Requirements for Coffee Brewers 1. Coffee Volume: The volume of the brew basket must be sized in proportion
Certified Home Brewer Program Minimum Certification Requirements SCA's Minimum Certification Requirements for Coffee Brewers 1. Coffee Volume: The volume of the brew basket must be sized in proportion
Shades from Shapes. Materials Required. Task 1: Movement of Particles
 Vigyan Pratibha Learning Unit Shades from Shapes Materials Required Task 1: Beaker, water, ink, etc. Task 2: Wheat flour, tap water, food colour powder (green or red) available with grocer, a bowl (for
Vigyan Pratibha Learning Unit Shades from Shapes Materials Required Task 1: Beaker, water, ink, etc. Task 2: Wheat flour, tap water, food colour powder (green or red) available with grocer, a bowl (for
involves separating solid in liquid mixtures where the solid particles are large, such as vegetables in water, where you want to retrieve the solid.
 A mixture is formed when two or more substances are mixed physically and not chemically combined. These substances can be separated and recovered again by physical and not chemical means, although not
A mixture is formed when two or more substances are mixed physically and not chemically combined. These substances can be separated and recovered again by physical and not chemical means, although not
Winemaking and Sulfur Dioxide
 Winemaking and Sulfur Dioxide Prepared and Presented by: Frank Schieber, Amateur Winemaker MoundTop MicroVinification Vermillion, SD www.moundtop.com schieber@usd.edu Outline: Sulfur Dioxide (Free SO 2
Winemaking and Sulfur Dioxide Prepared and Presented by: Frank Schieber, Amateur Winemaker MoundTop MicroVinification Vermillion, SD www.moundtop.com schieber@usd.edu Outline: Sulfur Dioxide (Free SO 2
Smart Valve Cold Brew Coffee Maker { Instruction Manual
 Smart Valve Cold Brew Coffee Maker { Instruction Manual Table of Contents Important Safeguards... 2 Glass Decanter Safety Precautions... 3 Getting to Know Your Cold Brew Coffee Maker... 4 Easy-Grab Tab
Smart Valve Cold Brew Coffee Maker { Instruction Manual Table of Contents Important Safeguards... 2 Glass Decanter Safety Precautions... 3 Getting to Know Your Cold Brew Coffee Maker... 4 Easy-Grab Tab
Objective: Decompose a liter to reason about the size of 1 liter, 100 milliliters, 10 milliliters, and 1 milliliter.
 NYS COMMON CORE MATHEMATICS CURRICULUM Lesson 9 3 2 Lesson 9 Objective: Decompose a liter to reason about the size of 1 liter, 100 milliliters, 10 milliliters, and 1 milliliter. Suggested Lesson Structure
NYS COMMON CORE MATHEMATICS CURRICULUM Lesson 9 3 2 Lesson 9 Objective: Decompose a liter to reason about the size of 1 liter, 100 milliliters, 10 milliliters, and 1 milliliter. Suggested Lesson Structure
Method 3 (carbon dioxide)
 Method 3 (carbon dioxide) Aim: Observing the production of carbon dioxide gas from chemical raising agents. Equipment Digital scales 5 measuring jugs or tall glasses Kettle Additional measuring jug Digital
Method 3 (carbon dioxide) Aim: Observing the production of carbon dioxide gas from chemical raising agents. Equipment Digital scales 5 measuring jugs or tall glasses Kettle Additional measuring jug Digital
Grapes of Class. Investigative Question: What changes take place in plant material (fruit, leaf, seed) when the water inside changes state?
 Grapes of Class 1 Investigative Question: What changes take place in plant material (fruit, leaf, seed) when the water inside changes state? Goal: Students will investigate the differences between frozen,
Grapes of Class 1 Investigative Question: What changes take place in plant material (fruit, leaf, seed) when the water inside changes state? Goal: Students will investigate the differences between frozen,
Please be sure to save a copy of this activity to your computer!
 Thank you for your purchase Please be sure to save a copy of this activity to your computer! This activity is copyrighted by AIMS Education Foundation. All rights reserved. No part of this work may be
Thank you for your purchase Please be sure to save a copy of this activity to your computer! This activity is copyrighted by AIMS Education Foundation. All rights reserved. No part of this work may be
St.Mary s Catholic High School-Dubai Name YEAR 5 SCIENCE REVISION WORKSHEET
 St.Mary s Catholic High School-Dubai Date..Jan,2018 Name YEAR 5 SCIENCE REVISION WORKSHEET (LIFE CYCLES of plants and animals) 1. What organ does a plant need to reproduce?.. 2. In which part of the flower
St.Mary s Catholic High School-Dubai Date..Jan,2018 Name YEAR 5 SCIENCE REVISION WORKSHEET (LIFE CYCLES of plants and animals) 1. What organ does a plant need to reproduce?.. 2. In which part of the flower
Operating the Rancilio Silvia after PID kit modification Version 1.1
 Operating the Rancilio Silvia after PID kit modification Version 1.1 When the machine is turned on, the controller will display the boiler temperature in the machine. The temperature reading will start
Operating the Rancilio Silvia after PID kit modification Version 1.1 When the machine is turned on, the controller will display the boiler temperature in the machine. The temperature reading will start
Dry Ice Color Show Dry Ice Demonstrations
 Dry Ice Color Show Dry Ice Demonstrations SCIENTIFIC Introduction Add a small piece of solid carbon dioxide to a colored indicator solution and watch as the solution immediately begins to boil and change
Dry Ice Color Show Dry Ice Demonstrations SCIENTIFIC Introduction Add a small piece of solid carbon dioxide to a colored indicator solution and watch as the solution immediately begins to boil and change
Bag-In-Box Package Testing for Beverage Compatibility
 Bag-In-Box Package Testing for Beverage Compatibility Based on Proven Plastic Bottle & Closure Test Methods Standard & Analytical Tests Sensory evaluation is subjective but it is the final word or approval.
Bag-In-Box Package Testing for Beverage Compatibility Based on Proven Plastic Bottle & Closure Test Methods Standard & Analytical Tests Sensory evaluation is subjective but it is the final word or approval.
The dissociation of a substance in hot water can be well described by the diffusion equation:
 In this article we explore and present our solution to problem no. 15 of the 2017 International Physicist Tournament, Tea with honey. First we present our idea for the solution to the problem, then our
In this article we explore and present our solution to problem no. 15 of the 2017 International Physicist Tournament, Tea with honey. First we present our idea for the solution to the problem, then our
(a) Dead-end/conventional filtration fluid flow perpendicular to the filter medium. (b) Crossflow filtration fluid flow parallel to the filter
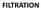 FILTRATION (a) Dead-end/conventional filtration fluid flow perpendicular to the filter medium. (b) Crossflow filtration fluid flow parallel to the filter medium. Filtration Generally carry out in the early
FILTRATION (a) Dead-end/conventional filtration fluid flow perpendicular to the filter medium. (b) Crossflow filtration fluid flow parallel to the filter medium. Filtration Generally carry out in the early
Prac;cal Sessions: A step by step guide to brew recipes Milk for baristas
 AGENDA: An overview of the Barista Modules. Who they are aimed at? How does the learning and teaching develop from Founda@on through to Professional Updates on the current exams & other work underway Feedback:
AGENDA: An overview of the Barista Modules. Who they are aimed at? How does the learning and teaching develop from Founda@on through to Professional Updates on the current exams & other work underway Feedback:
Winemaking and Tartrate Instability
 Winemaking and Tartrate Instability (Revised 9/19/2011) Prepared and Presented by: Frank Schieber, Amateur Winemaker MoundTop MicroVinification Vermillion, SD www.moundtop.com schieber@usd.edu Tartrate
Winemaking and Tartrate Instability (Revised 9/19/2011) Prepared and Presented by: Frank Schieber, Amateur Winemaker MoundTop MicroVinification Vermillion, SD www.moundtop.com schieber@usd.edu Tartrate
Dry Ice Rainbow of Colors Weak Acids and Bases
 Dry Ice Rainbow of Colors Weak Acids and Bases SCIENTIFIC Introduction Add a small piece of solid carbon dioxide to a colored indicator solution and watch as the solution immediately begins to boil and
Dry Ice Rainbow of Colors Weak Acids and Bases SCIENTIFIC Introduction Add a small piece of solid carbon dioxide to a colored indicator solution and watch as the solution immediately begins to boil and
VWT 272 Class 10. Quiz 9. Number of quizzes taken 24 Min 11 Max 30 Mean 26.5 Median 28 Mode 30
 VWT 272 Class 10 Quiz 9 Number of quizzes taken 24 Min 11 Max 30 Mean 26.5 Median 28 Mode 30 Lecture 10 Some Chemical Structures and the Sulfur Dioxide Family The difference between professional winemakers
VWT 272 Class 10 Quiz 9 Number of quizzes taken 24 Min 11 Max 30 Mean 26.5 Median 28 Mode 30 Lecture 10 Some Chemical Structures and the Sulfur Dioxide Family The difference between professional winemakers
How Do Leaves Breath?
 How Do Leaves Breath? A wonderful visual experiment that shows how leaves actually produce oxygen from photosynthesis. This experiment can be done in your home with materials that you may find in your
How Do Leaves Breath? A wonderful visual experiment that shows how leaves actually produce oxygen from photosynthesis. This experiment can be done in your home with materials that you may find in your
FOSS NOTEBOOK CHEMICAL INTERACTIONS
 FOSS NOTEBOOK CHEMICAL INTERACTIONS Investigation #7: Phase Change *BIG QUESTION: What conditions induce substances to change from one phase to another?* Is It Melting? The list below involves situations
FOSS NOTEBOOK CHEMICAL INTERACTIONS Investigation #7: Phase Change *BIG QUESTION: What conditions induce substances to change from one phase to another?* Is It Melting? The list below involves situations
LAB: One Tube Reaction Part 1
 AP Chemistry LAB: One Tube Reaction Part 1 Objective: To monitor and document the chemical changes occurring in a single test tube containing a predetermined mixture of chemicals. Materials: test tube,
AP Chemistry LAB: One Tube Reaction Part 1 Objective: To monitor and document the chemical changes occurring in a single test tube containing a predetermined mixture of chemicals. Materials: test tube,
Roasting For Flavor. Robert Hensley, 2014 SpecialtyCoffee.com Page 1 of 7 71 Lost Lake Lane, Campbell, CA USA Tel:
 One of the wonderful things about coffee is how responsive it is to all the nuances and variations in growing, processing, roasting and brewing. In the roasting especially, these touches have a magic all
One of the wonderful things about coffee is how responsive it is to all the nuances and variations in growing, processing, roasting and brewing. In the roasting especially, these touches have a magic all
Chapter 4. Basic Principles of Cooking and Food Science. Copyright 2011 by John Wiley & Sons, Inc. All Rights Reserved
 Chapter 4 Basic Principles of Cooking and Food Science Copyright 2011 by John Wiley & Sons, Inc. All Rights Reserved No written recipe can be 100 percent accurate. The judgment of the cook is still the
Chapter 4 Basic Principles of Cooking and Food Science Copyright 2011 by John Wiley & Sons, Inc. All Rights Reserved No written recipe can be 100 percent accurate. The judgment of the cook is still the
May 28, Final Exam Review.notebook
 A man is preparing two solutions of salt dissolved in water. Solution "A" has 15 grams of salt dissolved in 100 ml of water. Solution "B", which also has 100 ml of water, is less concentrated than solution
A man is preparing two solutions of salt dissolved in water. Solution "A" has 15 grams of salt dissolved in 100 ml of water. Solution "B", which also has 100 ml of water, is less concentrated than solution
Properties of Water. reflect. look out! what do you think?
 reflect Water is found in many places on Earth. In fact, about 70% of Earth is covered in water. Think about places where you have seen water. Oceans, lakes, and rivers hold much of Earth s water. Some
reflect Water is found in many places on Earth. In fact, about 70% of Earth is covered in water. Think about places where you have seen water. Oceans, lakes, and rivers hold much of Earth s water. Some
An Investigation into the relative gluten content of wheat flours
 An Investigation into the relative gluten content of wheat flours By Abbey.Kumar Student Number: 170312 Mrs Hendriks Background Research Earlier this year, my younger cousin was diagnosed with coeliac
An Investigation into the relative gluten content of wheat flours By Abbey.Kumar Student Number: 170312 Mrs Hendriks Background Research Earlier this year, my younger cousin was diagnosed with coeliac
The Cranberry. Sample file
 The Cranberry MATERIALS: THINGS YOU NEED A package of fresh cranberries (six cranberries for each student); a pin; a sharp knife, a ruler, white paper, a glass, water, 2 bowls. LABORATORY WORK 1. Pick
The Cranberry MATERIALS: THINGS YOU NEED A package of fresh cranberries (six cranberries for each student); a pin; a sharp knife, a ruler, white paper, a glass, water, 2 bowls. LABORATORY WORK 1. Pick
Sticking and mold control. TIA Tech 2017 Los Angeles, California Steve Bright
 Sticking and mold control TIA Tech 2017 Los Angeles, California Steve Bright Sticking Package Sticking Defined: Two or more tortillas that will not separate from each other without tearing or ripping after
Sticking and mold control TIA Tech 2017 Los Angeles, California Steve Bright Sticking Package Sticking Defined: Two or more tortillas that will not separate from each other without tearing or ripping after
