NIH Public Access Author Manuscript Semin Immunopathol. Author manuscript; available in PMC 2013 July 01.
|
|
|
- Julian Thompson
- 5 years ago
- Views:
Transcription
1 NIH Public Access Author Manuscript Published in final edited form as: Semin Immunopathol July ; 34(4): doi: /s y. Animal Models to Study Gluten Sensitivity 1 Eric V. Marietta, PhD 1,2,3 and Joseph A. Murray, MD 1,2 1 Department of Immunology, Mayo Clinic, Rochester, Minnesota 2 Department of Gastroenterology and Hepatology, Mayo Clinic, Rochester, Minnesota 3 Department of Dermatology, Mayo Clinic, Rochester, Minnesota Abstract The initial development and maintenance of tolerance to dietary antigens is a complex process that, when prevented or interrupted, can lead to human disease. Understanding the mechanisms by which tolerance to specific dietary antigens is attained and maintained is crucial to our understanding of the pathogenesis of diseases related to intolerance of specific dietary antigens. Two diseases that are the result of intolerance to a dietary antigen are celiac disease (CD) and dermatitis herpetiformis (DH). Both of these diseases are dependent upon the ingestion of gluten (the protein fraction of wheat, rye, and barley) and manifest in the gastrointestinal tract and skin, respectively. These gluten-sensitive diseases are two examples of how devastating abnormal immune responses to a ubiquitous food can be. The well-recognized risk genotype for both is conferred by either of the HLA class II molecules DQ2 or DQ8. However, only a minority of individuals who carry these molecules will develop either disease. Also of interest is that the age at diagnosis can range from infancy to years of age. This would indicate that intolerance to gluten may potentially be the result of two different phenomena. The first would be that, for various reasons, tolerance to gluten never developed in certain individuals, but that for other individuals, prior tolerance to gluten was lost at some point after childhood. Of recent interest is the concept of non-celiac gluten sensitivity, which manifests as chronic digestive or neurologic symptoms due to gluten, but through mechanisms that remain to be elucidated. This review will address how animal models of gluten-sensitive disorders have substantially contributed to a better understanding of how gluten intolerance can arise and cause disease. Introduction While humans by and large tolerate a vast array of dietary antigens without negative consequences, intolerances do occur. Celiac disease (CD) and its skin manifestation, dermatitis herpetiformis (DH), are two examples of enteric intolerance toward a dietary antigen. Both diseases are characterized by the development of enteropathy after the ingestion of gluten, which is a group of proteins found in wheat, barley, and rye [1, 2]. The development of this intolerance may result from either a failure in the initial development of tolerance to gluten or the loss of tolerance at some point after tolerance to gluten has been initially established. To better understand the immunologic pathways and mechanisms that inhibit the generation of tolerance to gluten or the loss of tolerance to gluten in adults, there are many different animal models of gluten sensitivity that can be used (Figure 1). These models utilize three primary species, dogs, monkeys, and mice, although a few studies on gluten sensitivity have been done with other species (eg, rabbits and rats) [3]. The rat model has been a useful 1 This article is published as part of the Special Issue on Celiac Disease [34:5]
2 Marietta and Murray Page 2 model for gluten digestion and studying the in vivo effects of gliadin on enterocytes [4, 5]. The dog and nonhuman primate models are both spontaneous models of CD, while mouse models are not spontaneous and need gliadin sensitization, chemical and/or drug treatment, and genetic alterations in order to develop features of CD. However, with mice there is a great advantage over the other models in that transgenes can be introduced in order to evaluate the contribution of specific genes to the development of tolerance to gluten. Although every model has certain elements of CD, not all elements of CD have been incorporated into one model yet. Depicted in Table 1 are the four prominent animal species used for modeling gluten sensitivity and which elements are present in each model. This separation of elements allows us to understand the interplay and effect that each element has on the final manifestation of disease. Currently there are two well-known animal models that spontaneously generate glutendependent diarrhea, the dog and the rhesus macaque (Table 1). A recent publication suggests that gluten dependent colic spontaneously develops in horses, making it a third spontaneous model of gluten sensitivity [6]. Common to all three of these spontaneous models is the lack of an association with MHC II. With the dog model, a rigorous study concluded that there definitely was no association with the MHC II, and so far no published reports have addressed this with either the rhesus macaque model or the horse model. All of the mouse models that incorporate celiac-associated MHC II alleles (DQ2 and DQ8), in contrast, do not spontaneously develop gluten-dependent enteropathy. These results bring up the question of how intolerance to gluten develops. One possibility is that genetically susceptible individuals never develop tolerance to gluten. The second possibility is that genetically susceptible individuals are exposed to an environmental influence that causes a loss of tolerance to gluten. All of the animal models listed in Table 1 provide clues as to whether either possibility may be present in celiac patients. Only one study has been published on the horse model, and in that study, one horse was identified that had substantially higher levels of IgA against tissue transglutaminase (ttg) and IgA against horse endomysium (EMA) than any of the other horses tested[6]. These levels were significantly decreased after 6 months on a gluten free diet. Similarly, the shortened villi were found to be markedly increased in length after the 6 month gluten-free diet. With only one horse currently identified as celiac-like, much more work needs to be done to determine what other elements of celiac disease are present in this model. With the dog model, Irish Setters develop wheat-dependent partial-villous atrophy with intraepithelial lymphocyte infiltration that is genetically transferred but not MHC II dependent, and so this model most resembles celiac infants and potentially what occurs in the initial stages of pathogenesis in celiac infants [7 9]. It is noteworthy that the Irish Setter model did not have increased levels of antigliadin antibodies while on a wheat-containing diet, indicating a purely innate (nonadaptive) response to gliadin (gluten) in these animals [10]. This latter point supports the theory that the CD4+ T-cell response to gluten and gliadin may be triggered by or enabled by a strong aberrant innate response to gluten. With the nonhuman primate (rhesus macaque) model, a subset of monkeys will develop gluten-dependent enteropathy. This enteropathy consists of partial-villous atrophy, increased intraepithelial lymphocytes (IELs), and antigliadin and anti-ttg antibodies. To identify these individuals, juvenile monkeys (<4 years of age) were first screened for the development of clinical symptoms of noninfectious, chronic diarrhea of idiopathic origin [11, 12]. The diarrheic monkeys were then screened for the development of both antigliadin and anti-ttg antibodies. Those that were positive for both were then evaluated by endoscopy and found to have villous atrophy that reversed with a gluten-free diet. In contrast to the dog model, however, a skin rash similar to DH was observed in the rhesus
3 Marietta and Murray Page 3 Role of CD4+ T cells Role of MHC II model [13]. This dermatitis resolved with a gluten-free diet and had IgA deposits that were ttg+. Co-localization of epidermal transglutaminase with the IgA deposits was not evaluated. It was also not determined if these animals had villous atrophy or increased numbers of IELs in the intestinal epithelium. However, recent publications have shown that the enteropathy in the diarrheic rhesus macaques has many similarities to the innate immune response to gliadin as part of CD in humans. By using healthy controls as a comparison, it was shown that the 33 amino acid gliadin-derived peptide (33mer) could cross the epithelial barrier only in monkeys that displayed celiac-like enteropathy. This gluten-dependent intestinal permeability was not found to occur in healthy controls, suggesting that gliadininduced intestinal permeability is an aberrant response to gliadin and may precede the development of the focused CD4+ T-cell response to gliadin. Indeed, it is not entirely clear whether the gluten-sensitive enteropathy in the nonhuman primate model is mediated by CD4+ T cells, and, in this respect, is similar to the dog model. If this is true, this result further supports the theory that there can be situations where a gluten-dependent enteropathy occurs in the absence of a CD4+ T-cell response. While the large animal models may more closely mimic the innate immune responses to gluten, the central role of CD4+ T cells in causing enteropathy has been studied in an adaption of the T-cell transfer model of colitis [14, 15] (Table 1). In this study, C57BL/6 donor mice were raised on a gluten-free chow (GFC) and injected at the base of the tail with gliadin in complete Freund s adjuvant (CFA) and CD4+ CD45RBlow CD25 T cells were subsequently isolated from the spleens. These cells were then injected intraperitoneally into Rag1 / recipient mice. This resulted in crypt hyperplasia and villous atrophy when the recipient mice were orally challenged with gluten. Interestingly, this developed primarily in the duodenum and with increased severity in the proximal jejunum, but rarely in the ileum, similar to human CD [16]. When the recipient mice were placed back onto a GFC, these pathologies disappeared, demonstrating the gluten dependence of the pathology. These results also demonstrated that CD4+ T cells (or at least a subset of them) could mediate gluten-dependent intestinal damage. However, as this mouse model used C57BL/6 mice, the model was dependent upon mouse MHC II and therefore could not address the role of DQ2 and DQ8. To address the role of MHC II (specifically DQ8), Black et al [17] used a transgenic mouse that expressed a genomic fragment that contained the celiac-predisposing gene of DQ8 (Table 1). Without sensitization, they did not develop symptoms; however, once sensitized to gluten by intraperitoneal injection with gluten, they did develop a strong T-cell proliferative response as well as an anti gliadin antibody response [17]. A later study with these mice demonstrated that with an intraperitoneal injection with gliadin and an additional 3 weeks of gavage with gliadin, an increase in the number of CD3+ IELs developed in the absence of villous atrophy [18] (Table 1). There was also a gliadin-dependent neuromuscular dysfunction that consisted of altered muscle contraction and ion transport. Studies with transgenic mice that expressed DQ2 with DR3 had similar results, with strong T-cell proliferation to gliadin after sensitization to gliadin, but no development of enteropathy [19]. Thus, DQ2 and DQ8 are necessary but insufficient alone for the development of clinical disease. Despite the lack of development of substantial villous atrophy, the DQ8 transgenic mouse has provided us with great insight as to why a majority of celiac patients have T-cell responses against deamidated epitopes of gliadin. In the one study by Hovhannisyan et al
4 Marietta and Murray Page 4 [20], it was shown that the B57 polymorphism of DQ8, which lacks an Aspartic acid at the B57 position of DQ8, allowed for the native form of the immunogenic gliadin epitope to bind to DQ8. It was also demonstrated that this very same polymorphism that allowed for binding to the native form of the gliadin epitope also allowed for the binding of deamidated gliadin epitopes and actually resulted in a heteroclitic (stronger) response to the deamidated form of the gliadin epitiope. This latter observation may explain why pediatric celiac cases have T-cell responses against a number of gluten-derived epitopes, but a substantial number of adult cases have T-cell responses predominantly against deamidated gliadin epitopes [21]. Role of Tissue Transglutaminase (ttg) Another arm of the immune system that is associated with the development of CD is the B cell, characterized in humans by the presence of antibodies directed against self and antibodies directed against gluten, both of which are widely used for detection. Many studies have demonstrated that anti-ttg antibodies (predominantly IgA, but also IgG) are closely associated with the development of gluten-sensitive enteropathy; however, it is not clear why these antibodies are produced or even if they have a pathogenic role in the development of CD. One study found that these antibodies are deposited in the small intestine of untreated celiac patients [22] and another study observed that some celiac patients have antittg antibodies that may disrupt or block the enzymatic function of ttg [23]. It is the production of these antibodies that suggests that CD may be an autoimmune disease and demonstrates that not only tolerance against a dietary protein, but also tolerance against a self-antigen, is disrupted in celiac patients. However, the dependence of these antibodies and the disease itself on the continued exposure to an exogenous antigen places CD in a unique position in that it is the only autoimmune disease for which we have detected the exogenous trigger. Animal models have provided information into the pathogenic role of these antibodies in both CD and DH. A direct analysis of the pathogenic properties of antibodies in DH was addressed using a passive transfer mouse model [24]. This was based on a passive transfer model that the authors had generated for linear IgA bullous disease (LABD). In a previous publication, the authors had determined that in a common subtype of LABD, patients produced IgA that bound to a 97-kDa protein (LABD-97) found in epidermal extracts [25]. Using athymic mice and SCID mice grafted with human skin, the authors demonstrated that purified IgA or IgG antihuman LABD97 injected into these mice resulted in deposits at the basement membrane zone of the human skin grafts, and thereby mimicked the LABD pathology. By contrast, sera from DH patients did not provide granular IgA deposits at the basement membrane zone that are typically found in DH patients [24]. Undaunted, Dr Zone s group [26] improved this model by later transferring purified antibodies against epidermal transglutaminase into these mice. They found that granular IgA deposits did form at the basement membrane zone with this antibody, supporting the theory that had been earlier proposed by Sardy et al [27], that anti-tge antibodies comprise the IgA deposits in DH. However, no separation of the dermal/epidermal junction developed as had with the linear IgA model. With the passive transfer model designed by Dr Zone, we see that DH sera, which contains both anti-epidermal transglutaminase and antitissue transglutaminase antibodies, did not result in the production of gluten-dependent blistering or enteropathy; albeit, the transfer of purified anti-tge did result in antibody deposition in the epidermal/ dermal junction. To address the possibility of anti-ttg antibodies mediating damage in the intestine, a number of studies used different approaches to overexpress anti-ttg antibodies in vivo. One group induced the expression of anti-ttg by using recombinant adeno-associated virus (raav) and a sequence for antihuman ttg derived from a phage display library of
5 Marietta and Murray Page 5 antibodies from intestinal lymphocytes of a celiac patient [28] (Table 1). The Fc domains of mouse IgG1 were used to make the final mini-antibody (MB) suitable for in vivo studies in mice, and anti-ttg MB raav virions were injected into C57BL6 mice [29]. Four weeks after injection, a substantial amount of anti-ttg MB was detected in the sera, and deposits were found in the muscle tissue that was injected, but not in the uninjected contralateral muscle tissue. No deposits were detected in the intestine and no increase in intestinal permeability or clinical symptoms associated with CD were observed to develop in these mice, despite the presence of deposits in the lung and corresponding granulomatous structures. These results would indicate that anti-ttg antibodies by themselves do not trigger the development of enteropathy and, more interestingly, do not bind to small intestinal tissue, suggesting that other phenomena are required for the anti-ttg antibodies to bind to the small intestinal tissue of the mouse. In a human study, blocking the activity of ttg with antibodies against ttg in vitro decreased gliadin-specific activation of T-cell clones derived from the small intestines of celiac patients [30], suggesting that ttg was necessary for the development of glutendependent enteropathy. Mice that overexpress IL15 with the enterocyte-specific T3b promoter and develop enteropathy in a gluten-independent fashion do have a considerable amount of anti-ttg IgA produced [31]. All of these results would suggest, then, that antittg antibodies are produced after (or simultaneously with) the development of small intestinal inflammation, and can develop in the absence of a gluten-driven CD4+ T-cell response. Of interest was one study that isolated antibodies that bound to transglutaminase 2 (TG2/ ttg) [32]. The authors found that there were two classes; class I only bound to ttg, whereas class II bound to ttg, transglutaminase 3 (TG3), and transglutaminase 6 (TG6). TG3 is an epidermal transglutaminase, which is expressed in the skin, and TG6 is a transglutaminase that is expressed in the brain. Adult C57Bl/6 mice were injected intraventricularly with purified single-chain variable fragments (scfvs) that were derived from the blood of celiac patients and fell into the two classes of transglutaminase binding [32]. Both classes caused dramatic motor impairment in the mice and were found to localize to the brains of the mice, specifically localizing to the lateral ventricles the collicoli in the cerebellum, and the brain parenchyma. However, it was not reported whether any of the injected scfvs localized to the small intestinal tissue, or if gluten affected the development of disease. Genetic Predisposition Toward Autoimmunity These results, with mouse models of CD, would suggest then that a strong CD4+ T-cell response against gliadin and a strong B-cell response to ttg are not sufficient by themselves to cause gluten-dependent enteropathy. Because CD is associated with other autoimmune diseases [33], it is likely that genes that contribute toward the development of autoimmunity are required for the development of the gluten-sensitive diseases; this theory is supported by the finding that DQ2 and DQ8 only provide 30% to 35% of the familial risk for developing CD [34]. Many of the non-hla candidate genes that have been identified in a Genomic Wide Association Study (GWAS) are involved in immune function, with some specifically associated with T-cell development of the thymus and other autoimmune diseases, such as type 1 diabetes (T1D) [35]. Other studies have shown that CD is tightly associated with T1D, such that celiac patients have over a two-fold greater risk of developing T1D (hazard ratio) [36]. Of interest is that T1D patients have a 20-fold increased risk for developing CD [37, 38]. Also, the mouse model oft1d, the non-obese diabetic (NOD) mouse, has reduced villous height and an increase in IELs while on a gluten-containing diet, further supporting the theory that many of the mechanistic pathways that contribute to T1D also contributes to the development of CD [39].
6 Marietta and Murray Page 6 Certainly MHC II is one of these mechanistic pathways that contribute to both diseases, since DQ2-DR3 and DQ8-DR4 haplotypes are associated with both diseases, although T1D is more associated with DQ8 and CD/DH are more associated with DQ2 [40, 41]. However, both diseases also share non-hla genes [35, 42]. Similar to CD, MHC II alone (found in IDDM1 in humans) is not sufficient for the development of T1D [43]. This has been demonstrated in the NOD mouse model in that the MHC II allele that predisposes toward the development of T1D, H-2 g7,-while required for disease development, cannot alone generate the development of T1D [44, 45]. Other genes are required to be present, and these have been designated Insulin-Dependent Diabetes (IDD) loci. Although most IDD loci only incrementally contribute to the development of diabetes, two IDD loci (5 and 13) were found to be shared between NOD mice and humans and contribute to the development of invasive insulitis [43]. An alternative interpretation is that the IDD loci contribute to a predisposition toward autoimmunity through a decreased ability to maintain tolerance to self, and thereby contribute to the development of other autoimmune diseases, such as CD. To test the hypothesis that autoimmune predisposing genes (genetic background) contribute to the development of gluten sensitivity, NOD mice were crossed with DQ8 transgenic mice. The resulting NOD Abo DQ8 mice are capable of developing tolerance to dietary gliadin and do not spontaneously develop the clinical symptoms of gluten-sensitive diseases [46] (Table 1). Similarly, the expression of the DQ2DR3 haplotype on the NOD background did not result in the spontaneous development of either gluten-sensitive disease [19]. These results would suggest then that even on a genetic background that predisposes toward the development of autoimmunity, the expression of DQ2 and DQ8 are still not sufficient to generate clinical symptoms of either gluten-sensitive disease. These results led to a very important conclusion, that something in addition to the combination of a genetic susceptibility to gluten sensitivity and to autoimmunity is required for the development of CD. Disruptions to Intestinal Homeostasis To address how disruptions to intestinal homeostasis might affect gluten sensitivity in these models, two groups used indomethacin, a COX inhibitor, which was shown to induce intestinal motility in rats [47], and later to transiently increase paracellular intestinal permeability in rats [48]. In the study that used transgenic mice that expressed HLA-DQ8, indomethacin treatment was administered in the drinking water (3 mg/100 ml) for 14 days after oral sensitization to gluten using cholera toxin [49], which consisted of gavaging the mice with gluten and cholera toxin simultaneously once every week for 3 weeks (Table 1). This resulted in decreased villous height without crypt hyperplasia and without an increase in IELs. The triple treatment also resulted in the increased production of IFNγ. In the study with transgenic mice that expressed both DQ8 and human CD4, gluten was injected intraperitoneally with acetic acid into the mice [50]. They were then intragastrically gavaged with 2 mg of gluten 3 times a week for 7 weeks. Twenty-four hours before each gluten gavage, indomethacin was administered by gavage (3.5 mg/kg). It was observed that gliadin plus indomethacin substantially increased the transcellular permeability over indomethacin alone, and that IFNγ production was considerably increased with treatment with both gliadin and indomethacin over either alone. Thus, although the two studies used different lines of transgenic mice and different sensitization protocols, they both came to the same conclusion: that indomethacin does not induce gluten-dependent villous atrophy with an increase in IEL numbers and crypt hyperplasia. Based on the results on gliadin-induced permeability with the rhesus macaque model, it may be that the gliadin-induced permeability that is associated with CD needs to be chronic, as opposed to transiently, induced, or that permeability is a co-phenomenon.
7 Marietta and Murray Page 7 Role of IL15 This theory that transient inductions of inflammation are not enough to induce a perpetuating CD4+ T-cell response to gliadin that results in enteropathy was further supported by a study that evaluated the use of polyinosinic/polycytidylic acid (poly [I:C]) with DQ2 transgenic mice. These mice were injected intraperitoneally with poly (I:C) to induce transient small intestinal damage that consisted of villous atrophy for 12 hours [51]. However, no T-cell proliferation that was gliadin specific was detected 72 hours after the poly (I:C) treatment and oral gliadin challenge. Similarly, treatment with methotrexate, which induces severe intestinal inflammation without villous atrophy for 3 to 5 days after treatment [52], also did not result in T-cell proliferation against gliadin, further supporting the findings with indomethacin, that transient disruptions to intestinal homeostasis through chemical means does not result in gluten-dependent enteropathy in genetically predisposed individuals. Celiac patients have increased levels of IL15 in the lamina propria [53], and IL15 has also been shown to disrupt the function of regulatory T cells in CD patients [54]. To address the role of IL15 in celiac disease, one study generated a mouse that overexpressed IL15 systemically (using a minimal MHC class I D d promoter) and crossed that with mice that expressed DQ8 [53] (Table 1). In this study, the authors found that the feeding of gliadin to mice maintained on a GFC resulted in an increase of CD3+ IELs to 30 per 100 enterocytes, which was 10-fold greater than the temporary disruption to intestinal homeostasis via anti- CD25 in the NOD Abo DQ8 mice [55]. Neither of the parent lines of mice (IL1-5 D d alone or DQ8 alone) had increased levels of IELs with gliadin feeding [53, 56]. This result would suggest that an increase in CD3+ IELs that is comparable to that found in celiac patients (>40/100 enterocytes) only occurs when there is an overexpression of IL15 in the lamina propria. However, the DQ8 D d IL15 transgenic mice did not develop villous atrophy. A different study inserted human IL15 behind an enterocyte specific promoter (T3b) (Table 1) [57]. This targeted overexpression of IL15 to the small intestine of mice resulted in inflammation in the small intestine, but not in the large intestine [57], with a villous heightto-crypt ratio of 2.1:1. There was also a marked increase (>10-fold) in CD8αβ + T cells in the small intestinal lamina propria [57]. Of considerable interest was the increased number of plasmablasts with corresponding increases in anti-ttg IgA, despite the lack of a glutenspecific CD4+ T-cell response [58]. Other autoantibodies that were produced were against double-stranded DNA and rheumatoid factor [58]. These results would suggest that the production of anti-ttg IgA and increase in infiltrating CD8+ T cells can all develop in the absence of a gluten-specific T-cell response. Since the promoter constructs for IL15 overexpression are different in the two studies (D d vs T3b), it would be of great interest to see if overexpression of IL15 by intestinal epithelial cells, such as the T3b driven expression of IL15, in a DQ8 transgenic (or DQ2/DR3) transgenic mouse model would result in the development of gluten-dependent villous atrophy. Role of regulatory T cells One study with NOD Abo DQ8 mice found that a partial depletion of regulatory T cells with injection of anti-cd25 led to an increase in CD3+ IELs and a lower villous-to-crypt ratio [54], both of which were made significant when gliadin sensitization was done after anti- CD25 treatment (P<0.01 and P<0.05, respectively). Much more dramatic was the severity of insulitis that developed with the anti-cd25 and (cholera toxin)/(gliadin sensitization). They did not, however, develop hyperglycemia [54]. These results suggested, then, that with a temporary but severe disruption to the maintenance of regulatory T cells
8 Marietta and Murray Page 8 (CD4+CD25+Foxp3+) in the NOD Abo DQ8 mice via anti-cd25 treatment, some features of gluten-dependent enteropathy developed temporarily [54]. Another study of gliadin-associated regulatory T cells generated a DQ2.5 transgenic mouse deficient in mouse MHC II (MHC II / ), in which a genomic fragment that contained DQ2.5 was used for microinjection, and crossed that with a transgenic mouse that expressed a gliadin-specific T cell receptor (TCR) [59] (Table 1). They then took CFSE-labeled CD4+ splenic T cells from the HLA-DQ2.gliadin-TCR.MHCII / double-transgenic mice and transferred them by intravenous injection into HLA-DQ2 recipient mice. These particular cells expressed both IFNγ and IL-10, but not FoxP3, and localized to the spleen after feeding of deamidated gliadin, as opposed to the mesenteric lymph nodes, as was observed in their ovalbumin/do11.10 control study. Of great interest was that the splenic CFSE CD4+ T cells in the recipient DQ2 mouse transferred a suppressive effect upon a gliadinspecific DTH response in the recipient HLA-DQ2 mice. This study would suggest that these Tr-1 like splenic cells may be another pathway to suppress inflammatory responses to deamidated gliadin. A different study demonstrated that CD4+CD25+FoxP3+ cells are completely functional in CD when IL15 has been removed through antibody depletion [56]. Thus, it remains to be seen if these splenic Tr-1 like cells are able to suppress inflammatory T-cell responses to deamidated gliadin in humans, especially in celiac patients and their family members. Role of Intestinal Microbiota in CD A number of papers have demonstrated that there are differences in the microbiota of untreated and treated celiac patients [60 62]. In addition, the treated CD patients also had differences with healthy controls [63]. Most of these differences have been compositional in that one bacterial species is increased in the overall percentage while others decreased. All of these studies showed that the proportion of Bacteroides and Escherichia coli species were increased in untreated celiac patients and the proportion of Bifidobacterium species decreased compared to both treated celiac patients and healthy controls. These changes raised the question of whether the bacteria that expand in untreated celiac patients are causing the disease or whether they are merely opportunistic and take advantage of the change in nutrients due to increased intestinal permeability. One study approached this question by taking germ-free rats raised and maintained on a GFC and administering the various bacterial species of interest [5]. The application of Bifidobacterium bifidum, gliadin, and IFNγ together to rat duodenal intestinal loops did not cause a translocation of bacteria. However, the application of E coli, gliadin, and IFNγ did result in the translocation of gliadin peptides across the epithelial layer. These results would indicate, then, that gliadin can translocate the epithelial barrier if certain bacterial species are present in greater proportions in the intestinal microbiota. Thus, it is possible that the composition of the intestinal microbiota plays a crucial role in the initial stages of gluten-dependent enteropathy, and may help explain why only a very small percentage of individuals develop gluten-dependent enteropathy. Exploring Gluten Dependence in Other Diseases As was discussed earlier, when the NOD ABo DQ8 mice are partially depleted of CD4+CD25 Foxp3+ regulatory cells and sensitized to gliadin, the mice develop insulitis [54]. This raises the question of whether gluten tolerance (and intolerance) plays a role in the development oft1d. The gluten connection to the development of T1D comes from the increased risk of developing both diseases. T1D patients have a 20-fold increased risk of developing CD [37, 38], and patients with CD have a 2.4-fold greater chance of developing T1D (hazard ratio) [36]. Although CD and its skin manifestation (DH) are clearly gluten-
9 Marietta and Murray Page 9 sensitive diseases (in that the inclusion of gluten in the diet has relatively immediate consequences), this review would be remiss if it did not cover the work that has been done with the effect of gluten upon the development of T1D in mice and rats. Currently, there are two spontaneous animal models of T1D, the NOD mouse and Bio-Breeding diabetes-prone (BBDP) rats. Of interest is that it has been clearly shown that raising both of these models on a GFC substantially delays the onset, as well reduces the overall incidence, of T1D compared to those raised on a standard gluten-containing chow (GCC) [39, 64 68]. Even the NOD mouse model displays elements of CD with increased numbers of IELs and decreased villous height when the mice are maintained on a GCC[39]. Although there is great controversy as to whether T1D patients are truly affected by dietary gluten, especially if a gluten-free diet can be beneficial for T1D patients, there is irrefutable evidence that dietary gluten does affect the development of diabetes in these two animal models [69, 70]. In both the NOD and BBDP animal models, it has been demonstrated that providing a glutencontaining diet to these animals will substantially increase the incidence of hyperglycemia [66]. However, it is also true that casein and other dietary proteins in the diet will also increase the incidence of diabetes in these animal models [71]. Therefore, if these animals are raised on a hydrolyzed diet, then the development of hyperglycemia is postponed [72, 73]. Clearly, these animal models display the classical symptom of intolerance to gluten in that the development of disease is affected by the consumption of gluten. The most important take-home message from this is that the introduction of gluten-derived protein into the diet triggers an autoimmune response against the islets, especially when regulatory T cells are diminished. A number of studies have focused on the potential of cross-reactivity, specifically focusing on a wheat-storage globulin protein found in wheat, called Glo-3. This was originally named Glb-1 and was identified using sera from diabetic BBDP rats [74]. It was later identified as Glo-3A [75]. Antibodies against Glb-1 (Glo-3A) were considerably higher in T1D patients than in controls [74]. It was later found that increased sera levels of zonulin, a marker for gut permeability in CD patients, DH patients, and T1D patients [76 78], were associated with increased sera levels of antibodies against Glb1 (Glo-3A) in humans using the Diabetes Autoimmunity Study in the Young (DAISY) [79]. Finally, antibodies against Glo-3A were purified from a patient with both CD and T1D using recombinant Glo-3A and then were used in a cross-reaction study using both rat jejunum and rat pancreas [80]. Interestingly, the antibodies enriched for Glo-3A binding bound to cells in the lamina propria of rat jejunum [80], specifically CD163+ macrophages; but they did not bind to rat pancreas. Thus, the presence of anti-glo-3a antibodies in T1D patients demonstrates that T1D patients mount an immune response to at least one wheat-derived protein. What is of great interest is the concept that T1D patients may be intolerant to gluten. This is certainly the case for the T1D patients that are later diagnosed with CD. The intriguing question is whether T1D patients as a whole group are intolerant to gluten or if it is only the one subset of T1D patients that later develops CD that is truly intolerant to gluten. The studies with Glo-3A demonstrate that T1D patients as a group produce an aberrant immune response to at least one wheat-derived protein. Also, the recent study (in 2011) with the partial depletion of CD4+CD25+Foxp3+ cells in gluten-sensitized NOD Abo DQ8 mice would suggest that the development of insulitis and gluten sensitivity are associated with each other [54]. Thus, although studies done on T1D patients have not demonstrated that gluten consumption affects the development of diabetes in humans, the BBDP rat, the NOD mouse, and the NOD Abo DQ8 mouse models all suggest that gluten does play a role in the development of T1D.
10 Marietta and Murray Page 10 Therapies to Establish Tolerance to Gluten If we proceed with the notion that clinical symptoms of CD can arise as either the inhibition of tolerance to gluten or the disruption/loss of tolerance to gluten, then individualized approaches should be taken when administrating therapies that target the different pathogenic steps (Figure 2). The best example of this would be the administration of therapies designed to re-establish tolerance to gluten-derived proteins (such as gliadin). One group did this by generating a strain of Lactococcus lactis to secrete an immunogenic epitope of gliadin [81]. This approach was based on a previous study in which Lactococcus lactis bacteria were successfully bioengineered to secrete IL-10 [82]. This study demonstrated that administration of this IL-10 secreting strain successfully ameliorated the development of colitis in IL-10 / mice and caused a 50% decrease in pathological symptoms in a Dextran Sulphate Sodium (DSS) model of colitis. Advancing this model further, the group bioengineered L lactis to secrete ovalbumin [83]. The ability to suppress in an antigen-specific fashion was shown using DO11.10 mice that express TCR specific for ovalbumin. The T-cell response was shown to be decreased via delayed-type hypersensitivity assay (DTH). This reduction was shown to be due to CD4+CD25 regulatory T cells expressing Foxp3 and CTLA4 via cell-transfer assays. Using the same approach, L lactis were bioengineered to secrete a deamidated form of a gliadin-derived epitope that was DQ8 restricted and immunogenic for CD [81]. The ability of this strain to induce tolerance to gliadin was evaluated using GFC weaned and maintained NOD Abo DQ8 mice. The gliadin-secreting, L lactis treated, GFC-weaned and maintained NOD Abo DQ8 mice were then parenterally sensitized to gliadin. The administration of gliadinsecreting L lactis successfully diminished the T-cell response to gliadin as evaluated by DTH [81]. Analysis of the production of cytokines in the intestine and the phenotype of T cells suggested that the bioengineered L lactis strain was inducing CD4+CD25 Foxp3+ T cells that were gliadin specific. A different study determined the efficacy of different probiotic strains on the development of gluten sensitivity in a DQ8 transgenic mouse model of gluten sensitivity [49]. In this study, the authors found that the administration of Lactobacillus fermentum, Bifidobacterium lactis, Lactobacillus plantarum, and Lactobacillus paracasei, along with gliadin and cholera toxin, intragastrically increased the expression of IFNγ and/or TNFα by means of mesenteric lymph nodes or splenic cells of DQ8 transgenic mice sensitized to gliadin. This result supported a previous finding, in which it was determined that the administration of Lactobacillus casei alone during gliadin sensitization to DQ8 transgenic mice with gliadin and cholera toxin had an adjuvant effect, resulting in an increased proliferative response to gliadin, with a corresponding increase in IFNγ production [81]. However, when Lactobacillus casei was administered to the DQ8 transgenic mice alone, in the absence of gliadin sensitization, IL-10 was increased, while IFNγ was not increased in the spleen and mesenteric lymph nodes [84]. Of interest was that when the group administered L case in a model of gluten-induced villous damage using gliadin, cholera toxin, and indomethacin, L casei then acted as a probiotic, and reduced villous blunting [85]. This result of reduced villous blunting was similar to that found with the administration of L casei to humans, wherein the small intestine was protected from damage induced by low doses of aspirin [86]. These latter results highlight the fact that chemical perturbations to the intestine will alter how the bacteria in the small intestinal microbiome will interact with the host s intestinal immune system. It would be reasonable to assume then that probiotic therapy in the absence of severe inflammation-inducing drugs would have a high chance of success in individuals who were diagnosed later in life, due presumably to a loss of tolerance to gliadin. However, the administration of such a therapy might not work in individuals who were diagnosed with CD as very young children, as they presumably never developed tolerance to gliadin.
11 Marietta and Murray Page 11 Another potentially curative therapy is the intradermal injection of immunogenic glutenderived peptides with the intent of generating tolerance to gluten. This approach was tested with HLA DQ2DR3 transgenic mice and resulted in a suppressed inflammatory CD4+ T- cell response to gliadin [87]. Yet another curative approach would be the administration of immunogenic gluten-derived peptides intranasally, as was successfully done with α-gliadin using DQ8 transgenic mice [88]. As with the bioengineered L lactis, these approaches probably would have a higher chance of success in individuals who once were tolerant to gluten, but lost it later in life, as opposed to individuals who never developed tolerance to gluten. It is also possible that tolerance to gluten will need to be maintained long term, as sensitivity to gluten may never abate. Therapies to Block T Cell Activation/Function The administration of products that only temporarily block celiac disease, such as polymeric blocking molecules, may prove to be effective in both groups of celiac patients. One study done with Abo DQ8 mice demonstrated that the administration of poly(hydroxyethylmethacrylate-co-styrene sulfonate) [P(HEMA-co-SS)], which complexes with gliadin in a relatively specific fashion, resulted in decreased numbers of CD3+ IELs and gliadin-induced barrier dysfunction [89]. As this approach temporarily blocks the activation of gliadin-specific T cells, this therapy would be expected to be effective in both groups of celiac patients (Figure 2). The use of peptidases that would render gliadin-derived peptides nonimmunogenic is another temporary therapy that may prove to be useful for both groups of celiac patients. One such example is a barley-derived endoprotease called EP-B2. The first animal model study that was done with EP-B2 used a rat model of gluten digestion [4]. In that model, they were able to demonstrate that the administration of EP-B2 resulted in a substantial decrease (~50%) in the release of the immunogenic 33mer in the small intestine. EP-B2 was later provided to gluten-sensitive rhesus macaques and shown to inhibit the development of clinical symptoms when the gluten-sensitive rhesus macaques were challenged with gluten [12]. Of concern, though, was that levels of anti-gliadin IgG and IgA and anti-ttg IgG were not decreased, and in fact were higher than the levels found in gluten-sensitive monkeys maintained on a gluten-containing diet [12]. Thus, although this therapy could theoretically be used for both individuals who never achieved tolerance to gliadin as well as individuals who had lost their tolerance to gluten, the potential for side effects due to ongoing inflammation is great. Therapies to Block Inflammation Induced by Epithelial Cells Another approach to temporarily blocking the inflammation would be the administration of antibodies against IL-15, the IL-15 receptor, or downstream (of IL-15) signaling molecules, in order to effectively block the signaling from the IL-15 receptor (Figure 2). Administration of anti-il15r to the mice that overexpressed IL-15 in the small intestine resulted in the reversal of villous atrophy and inflammation in the intestines of these mice [31]. Another approach was to target IL-15 itself, and this therapy was proven successful with the administration of the anti-human IL-15 antibody to the same line of mice that overexpressed IL-15 driven by the T3b promoter (enterocyte-specific) [90]. Targeting IL-15 or its receptor may prove to be quite useful in those celiac patients that express abnormally high levels of IL-15, as well as refractory celiac patients. A different approach to blocking the development of inflammatory processes is to block the tight junction disassembly that occurs in celiac patients on a gluten-containing diet [91] (Figure 2). This process has been shown to be due to the gliadin-induced release of zonulin and subsequent rearrangement of zonula occludens-1 (ZO-1) by epithelial cells, resulting in
12 Marietta and Murray Page 12 disruption of the tight junction assembly [91]. A recent paper has demonstrated that a novel drug called larazotide acetate, also known as AT1001, inhibits the redistribution and rearrangement of ZO-1 and actin, resulting in greater integrity in the tight junctions and less translocation of gliadin peptides [92]. This was determined in vitro using Caco-2 and IEC-6 cells and in vivo treatment of gliadin-sensitized hcd4dq8 transgenic mice with larazotide acetate and resulted in a decrease in macrophages in the small intestine and an improvement in barrier function parameters, including conductance and transcellular permeability [93]. Administration of this drug would be best if done at (or shortly before) the time of gluten ingestion, as was done with the hcd4dq8 transgenic mouse study, because the translocation of gliadin peptides occurs within 1 hour after gliadin is administered orally, as demonstrated by the rhesus macaque study [12]. Therapies Using Non-Immunogenic Wheat Strains Of course, there is also the approach of recognizing that a considerable number of celiac patients may be incapable of generating tolerance to gluten, because they never established it when they were infants. For these individuals, the best therapeutic approach may be to completely avoid the immunogenic peptides via the use of ancient strains of wheat. One study used DQ8 transgenic mice to evaluate the toxicity of the gluten-free grains (ie, tef, millet, amaranth, and quinoa) [94]. Mice were sensitized to gliadin by administration of cholera toxin and a peptic tryptic digest of gliadin, spleen, and mesenteric lymph nodes extracted and treated in vitro with a peptic tryptic digest of gliadin, tef, millet, amaranth, or quinoa. Only the peptic tryptic digest of gliadin generated a strong T-cell response, indicating that the four other grains did not have any immunogenic epitopes that crossreacted with the gliadin derived from Triticum aestivum (wheat). Similarly, only gliadin from T aestivum induced the production of IFNγ from seven different T-cell lines that were isolated from the small intestines of CD patients. These results would suggest that these four grains are safe for celiac patients to eat. Overall Conclusions Acknowledgments All of these studies with animal models have demonstrated some very important points. The most important point is that intolerance to gluten, as seen in CD and DH, is a conglomeration of both aberrant adaptive and aberrant innate immune responses to gluten. The second point is that the animal models would suggest that intolerance to gluten is initiated early in life, since both of the spontaneous animal models of CD have glutendependent villous atrophy, while none of the induced (in adulthood) rodent models do. Indeed, it is only with the introduction of the overexpressing IL-15 transgene into the DQ8 mouse model that the mouse models of gluten sensitivity achieve an element of spontaneous gluten-dependent enteropathy. This then comes to the third point, that the gluten-senstivie diseases are the result of a self-perpuated intertwining of the aberrent adaptive immune response to gluten with the aberrant innate response to gluten. How and when this intertwining occurs to result in gluten-dependent enteropathy (CD) or gluten-dependent pruritis (DH) will be the crucial questions to address next with the animal models of gluten sensitivity. This work was supported in part by NIH grant DK and Mayo Foundation for Medical Education and Research.
13 Marietta and Murray Page 13 Abbreviations CD DH T1D NOD IDD GFC GCC References celiac disease dermatitis herpetiformis type 1 diabetes non-obese diabetic insulin dependent diabetes gluten-free chow gluten-containing chow 1. Farrell RJ, Kelly CP. Celiac sprue. The New England journal of medicine. 2002; 346: /NEJMra [PubMed: ] 2. Fry L. Dermatitis herpetiformis: problems, progress and prospects. European journal of dermatology: EJD. 2002; 12: [PubMed: ] 3. March JB. High antigliadin IgG titers in laboratory rabbits fed a wheat-containing diet: a model for celiac disease? Dig Dis Sci. 2003; 48: [PubMed: ] 4. Gass J, Vora H, Bethune MT, Gray GM, Khosla C. Effect of barley endoprotease EP-B2 on gluten digestion in the intact rat. J Pharmacol Exp Ther. 2006; 318: /jpet [PubMed: ] 5. Cinova J, De Palma G, Stepankova R, Kofronova O, Kverka M, Sanz Y, Tuckova L. Role of intestinal bacteria in gliadin-induced changes in intestinal mucosa: study in germ-free rats. PLoS One. 2011; 6:e /journal.pone [PubMed: ] 6. van der Kolk JH, van Putten LA, Mulder CJ, Grinwis GC, Reijm M, Butler CM, von Blomberg BM. Gluten-dependent antibodies in horses with inflammatory small bowel disease (ISBD). Vet Q / Batt RM, Carter MW, McLean L. Morphological and biochemical studies of a naturally occurring enteropathy in the Irish setter dog: a comparison with coeliac disease in man. Research in veterinary science. 1984; 37: [PubMed: ] 8. Hall EJ, Batt RM. Dietary modulation of gluten sensitivity in a naturally occurring enteropathy of Irish setter dogs. Gut. 1992; 33: [PubMed: ] 9. Polvi A, Garden OA, Houlston RS, Maki M, Batt RM, Partanen J. Genetic susceptibility to gluten sensitive enteropathy in Irish setter dogs is not linked to the major histocompatibility complex. Tissue antigens. 1998; 52: [PubMed: ] 10. Hall EJ, Carter SD, Barnes A, Batt RM. Immune responses to dietary antigens in gluten-sensitive enteropathy of Irish setters. Research in veterinary science. 1992; 53: [PubMed: ] 11. Bethune MT, Borda JT, Ribka E, Liu MX, Phillippi-Falkenstein K, Jandacek RJ, Doxiadis GG, Gray GM, Khosla C, Sestak K. A non-human primate model for gluten sensitivity. PLoS One. 2008; 3:e /journal.pone [PubMed: ] 12. Bethune MT, Ribka E, Khosla C, Sestak K. Transepithelial transport and enzymatic detoxification of gluten in gluten-sensitive rhesus macaques. PLoS One. 2008; 3:e /journal.pone [PubMed: ] 13. Sestak K, Mazumdar K, Midkiff CC, Dufour J, Borda JT, Alvarez X. Recognition of Epidermal Transglutaminase by IgA and Tissue Transglutaminase 2 Antibodies in a Rare Case of <em>rhesus</em> Dermatitis. Journal of visualized experiments: JoVE / Powrie F, Leach MW, Mauze S, Menon S, Caddle LB, Coffman RL. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity. 1994; 1: [PubMed: ]
14 Marietta and Murray Page Freitag TL, Rietdijk S, Junker Y, Popov Y, Bhan AK, Kelly CP, Terhorst C, Schuppan D. Gliadinprimed CD4+CD45RBlowCD25 T cells drive gluten-dependent small intestinal damage after adoptive transfer into lymphopenic mice. Gut. 2009; 58: /gut [PubMed: ] 16. Dickey W, Hughes DF. Histology of the terminal ileum in coeliac disease. Scand J Gastroenterol. 2004; 39: / [PubMed: ] 17. Black KE, Murray JA, David CS. HLA-DQ determines the response to exogenous wheat proteins: a model of gluten sensitivity in transgenic knockout mice. J Immunol. 2002; 169: [PubMed: ] 18. Verdu EF, Huang X, Natividad J, Lu J, Blennerhassett PA, David CS, McKay DM, Murray JA. Gliadin-dependent neuromuscular and epithelial secretory responses in gluten-sensitive HLA-DQ8 transgenic mice. Am J Physiol Gastrointest Liver Physiol. 2008; 294:G /ajpgi [PubMed: ] 19. de Kauwe AL, Chen Z, Anderson RP, Keech CL, Price JD, Wijburg O, Jackson DC, Ladhams J, Allison J, McCluskey J. Resistance to celiac disease in humanized HLA-DR3-DQ2-transgenic mice expressing specific anti-gliadin CD4+ T cells. J Immunol. 2009; 182: / jimmunol [PubMed: ] 20. Hovhannisyan Z, Weiss A, Martin A, Wiesner M, Tollefsen S, Yoshida K, Ciszewski C, Curran SA, Murray JA, David CS, Sollid LM, Koning F, Teyton L, Jabri B. The role of HLA-DQ8 beta57 polymorphism in the anti-gluten T-cell response in coeliac disease. Nature. 2008; 456: /nature07524 [PubMed: ] 21. Vader W, Kooy Y, Van Veelen P, De Ru A, Harris D, Benckhuijsen W, Pena S, Mearin L, Drijfhout JW, Koning F. The gluten response in children with celiac disease is directed toward multiple gliadin and glutenin peptides. Gastroenterology. 2002; 122: [PubMed: ] 22. Korponay-Szabo IR, Halttunen T, Szalai Z, Laurila K, Kiraly R, Kovacs JB, Fesus L, Maki M. In vivo targeting of intestinal and extraintestinal transglutaminase 2 by coeliac autoantibodies. Gut. 2004; 53: [PubMed: ] 23. Esposito C, Paparo F, Caputo I, Rossi M, Maglio M, Sblattero D, Not T, Porta R, Auricchio S, Marzari R, Troncone R. Anti-tissue transglutaminase antibodies from coeliac patients inhibit transglutaminase activity both in vitro and in situ. Gut. 2002; 51: [PubMed: ] 24. Zone JJ, Egan CA, Taylor TB, Meyer LJ. IgA autoimmune disorders: development of a passive transfer mouse model. The journal of investigative dermatology Symposium proceedings/the Society for Investigative Dermatology, Inc [and] European Society for Dermatological Research. 2004; 9: /j x 25. Zone JJ, Taylor TB, Kadunce DP, Meyer LJ. Identification of the cutaneous basement membrane zone antigen and isolation of antibody in linear immunoglobulin A bullous dermatosis. J Clin Invest. 1990; 85: /JCI [PubMed: ] 26. Zone JJ, Schmidt LA, Taylor TB, Hull CM, Sotiriou MC, Jaskowski TD, Hill HR, Meyer LJ. Dermatitis herpetiformis sera or goat anti-transglutaminase-3 transferred to human skin-grafted mice mimics dermatitis herpetiformis immunopathology. J Immunol. 2011; 186: /jimmunol [PubMed: ] 27. Sardy M, Karpati S, Merkl B, Paulsson M, Smyth N. Epidermal transglutaminase (TGase 3) is the autoantigen of dermatitis herpetiformis. J Exp Med. 2002; 195: [PubMed: ] 28. Di Niro R, Ziller F, Florian F, Crovella S, Stebel M, Bestagno M, Burrone O, Bradbury AR, Secco P, Marzari R, Sblattero D. Construction of miniantibodies for the in vivo study of human autoimmune diseases in animal models. BMC biotechnology. 2007; 7: / [PubMed: ] 29. Di Niro R, Sblattero D, Florian F, Stebel M, Zentilin L, Giacca M, Villanacci V, Galletti A, Not T, Ventura A, Marzari R. Anti-idiotypic response in mice expressing human autoantibodies. Molecular immunology. 2008; 45: /j.molimm [PubMed: ] 30. Molberg O, McAdam S, Lundin KE, Kristiansen C, Arentz-Hansen H, Kett K, Sollid LM. T cells from celiac disease lesions recognize gliadin epitopes deamidated in situ by endogenous tissue
15 Marietta and Murray Page 15 transglutaminase. Eur J Immunol. 2001; 31: / (200105)31:5<1317::AID-IMMU1317>3.0.CO;2-I [PubMed: ] 31. Yokoyama S, Watanabe N, Sato N, Perera PY, Filkoski L, Tanaka T, Miyasaka M, Waldmann TA, Hiroi T, Perera LP. Antibody-mediated blockade of IL-15 reverses the autoimmune intestinal damage in transgenic mice that overexpress IL-15 in enterocytes. Proceedings of the National Academy of Sciences of the United States of America. 2009; 106: /pnas [PubMed: ] 32. Boscolo S, Lorenzon A, Sblattero D, Florian F, Stebel M, Marzari R, Not T, Aeschlimann D, Ventura A, Hadjivassiliou M, Tongiorgi E. Anti transglutaminase antibodies cause ataxia in mice. PLoS One. 2010; 5:e /journal.pone [PubMed: ] 33. Rashtak S, Marietta EV, Murray JA. Celiac sprue: a unique autoimmune disorder. Expert review of clinical immunology. 2009; 5: /eci [PubMed: ] 34. Schuppan D, Junker Y, Barisani D. Celiac disease: from pathogenesis to novel therapies. Gastroenterology. 2009; 137: /j.gastro [PubMed: ] 35. Dubois PC, Trynka G, Franke L, Hunt KA, Romanos J, Curtotti A, Zhernakova A, Heap GA, Adany R, Aromaa A, Bardella MT, van den Berg LH, Bockett NA, de la Concha EG, Dema B, Fehrmann RS, Fernandez-Arquero M, Fiatal S, Grandone E, Green PM, Groen HJ, Gwilliam R, Houwen RH, Hunt SE, Kaukinen K, Kelleher D, Korponay-Szabo I, Kurppa K, MacMathuna P, Maki M, Mazzilli MC, McCann OT, Mearin ML, Mein CA, Mirza MM, Mistry V, Mora B, Morley KI, Mulder CJ, Murray JA, Nunez C, Oosterom E, Ophoff RA, Polanco I, Peltonen L, Platteel M, Rybak A, Salomaa V, Schweizer JJ, Sperandeo MP, Tack GJ, Turner G, Veldink JH, Verbeek WH, Weersma RK, Wolters VM, Urcelay E, Cukrowska B, Greco L, Neuhausen SL, McManus R, Barisani D, Deloukas P, Barrett JC, Saavalainen P, Wijmenga C, van Heel DA. Multiple common variants for celiac disease influencing immune gene expression. Nat Genet. 2010; 42: /ng.543 [PubMed: ] 36. Ludvigsson JF, Ludvigsson J, Ekbom A, Montgomery SM. Celiac disease and risk of subsequent type 1 diabetes: a general population cohort study of children and adolescents. Diabetes Care. 2006; 29: /dc [PubMed: ] 37. Barera G, Bonfanti R, Viscardi M, Bazzigaluppi E, Calori G, Meschi F, Bianchi C, Chiumello G. Occurrence of celiac disease after onset of type 1 diabetes: a 6-year prospective longitudinal study. Pediatrics. 2002; 109: [PubMed: ] 38. Maki M, Hallstrom O, Huupponen T, Vesikari T, Visakorpi JK. Increased prevalence of coeliac disease in diabetes. Arch Dis Child. 1984; 59: [PubMed: ] 39. Maurano F, Mazzarella G, Luongo D, Stefanile R, D Arienzo R, Rossi M, Auricchio S, Troncone R. Small intestinal enteropathy in non-obese diabetic mice fed a diet containing wheat. Diabetologia. 2005; 48: /s [PubMed: ] 40. Sanjeevi CB, Sedimbi SK, Landin-Olsson M, Kockum I, Lernmark A. Risk conferred by HLA-DR and DQ for type 1 diabetes in 0-35-year age group in Sweden. Ann N Y Acad Sci. 2008; 1150: NYAS [pii] /annals [PubMed: ] 41. Jabri B, Sollid LM. Mechanisms of disease: immunopathogenesis of celiac disease. Nat Clin Pract Gastroenterol Hepatol. 2006; 3: ncpgasthep0582 [pii] /ncpgasthep0582 [PubMed: ] 42. Kagnoff MF. Celiac disease: pathogenesis of a model immunogenetic disease. J Clin Invest. 2007; 117: /JCI30253 [PubMed: ] 43. Fox CJ, Paterson AD, Mortin-Toth SM, Danska JS. Two genetic loci regulate T cell-dependent islet inflammation and drive autoimmune diabetes pathogenesis. Am J Hum Genet. 2000; 67: / [PubMed: ] 44. Rajagopalan G, Mangalam AK, Sen MM, Cheng S, Kudva YC, David CS. Autoimmunity in HLA- DQ8 transgenic mice expressing granulocyte/macrophage-colony stimulating factor in the beta cells of islets of Langerhans. Autoimmunity. 2007; 40: [pii] / [PubMed: ] 45. Fox CJ, Danska JS. Independent genetic regulation of T-cell and antigen-presenting cell participation in autoimmune islet inflammation. Diabetes. 1998; 47: [PubMed: ]
16 Marietta and Murray Page Marietta E, Black K, Camilleri M, Krause P, Rogers RS 3rd, David C, Pittelkow MR, Murray JA. A new model for dermatitis herpetiformis that uses HLA-DQ8 transgenic NOD mice. J Clin Invest. 2004; 114: /JCI21055 [PubMed: ] 47. Sababi M, Hallgren A, Nylander O. Interaction between prostanoids, NO, and VIP in modulation of duodenal alkaline secretion and motility. The American journal of physiology. 1996; 271:G [PubMed: ] 48. Nylander O, Hallgren A, Sababi M. COX inhibition excites enteric nerves that affect motility, alkaline secretion, and permeability in rat duodenum. Am J Physiol Gastrointest Liver Physiol. 2001; 281:G [PubMed: ] 49. D Arienzo R, Maurano F, Lavermicocca P, Ricca E, Rossi M. Modulation of the immune response by probiotic strains in a mouse model of gluten sensitivity. Cytokine. 2009; 48: / j.cyto [PubMed: ] 50. Natividad JM, Huang X, Slack E, Jury J, Sanz Y, David C, Denou E, Yang P, Murray J, McCoy KD, Verdu EF. Host responses to intestinal microbial antigens in gluten-sensitive mice. PLoS One. 2009; 4:e /journal.pone [PubMed: ] 51. Siegel M, Strnad P, Watts RE, Choi K, Jabri B, Omary MB, Khosla C. Extracellular transglutaminase 2 is catalytically inactive, but is transiently activated upon tissue injury. PLoS One. 2008; 3:e /journal.pone [PubMed: ] 52. de Koning BA, van Dieren JM, Lindenbergh-Kortleve DJ, van der Sluis M, Matsumoto T, Yamaguchi K, Einerhand AW, Samsom JN, Pieters R, Nieuwenhuis EE. Contributions of mucosal immune cells to methotrexate-induced mucositis. International immunology. 2006; 18: /intimm/dxl030 [PubMed: ] 53. DePaolo RW, Abadie V, Tang F, Fehlner-Peach H, Hall JA, Wang W, Marietta EV, Kasarda DD, Waldmann TA, Murray JA, Semrad C, Kupfer SS, Belkaid Y, Guandalini S, Jabri B. Co-adjuvant effects of retinoic acid and IL-15 induce inflammatory immunity to dietary antigens. Nature. 2011; 471: /nature09849 [PubMed: ] 54. Zanzi D, Stefanile R, Santagata S, Iaffaldano L, Iaquinto G, Giardullo N, Lania G, Vigliano I, Vera AR, Ferrara K, Auricchio S, Troncone R, Mazzarella G. IL-15 interferes with suppressive activity of intestinal regulatory T cells expanded in Celiac disease. The American journal of gastroenterology. 2011; 106: /ajg [PubMed: ] 55. Galipeau HJ, Rulli NE, Jury J, Huang X, Araya R, Murray JA, David CS, Chirdo FG, McCoy KD, Verdu EF. Sensitization to Gliadin Induces Moderate Enteropathy and Insulitis in Nonobese Diabetic-DQ8 Mice. J Immunol. 2011; 187: jimmunol [pii] / jimmunol [PubMed: ] 56. Fehniger TA, Suzuki K, Ponnappan A, VanDeusen JB, Cooper MA, Florea SM, Freud AG, Robinson ML, Durbin J, Caligiuri MA. Fatal leukemia in interleukin 15 transgenic mice follows early expansions in natural killer and memory phenotype CD8+ T cells. J Exp Med. 2001; 193: [PubMed: ] 57. Ohta N, Hiroi T, Kweon MN, Kinoshita N, Jang MH, Mashimo T, Miyazaki J, Kiyono H. IL-15- dependent activation-induced cell death-resistant Th1 type CD8 alpha beta+nk1.1+ T cells for the development of small intestinal inflammation. J Immunol. 2002; 169: [PubMed: ] 58. Yokoyama S, Takada K, Hirasawa M, Perera LP, Hiroi T. Transgenic mice that overexpress human IL-15 in enterocytes recapitulate both B and T cell-mediated pathologic manifestations of celiac disease. Journal of clinical immunology. 2011; 31: /s [PubMed: ] 59. Du Pre MF, Kozijn AE, van Berkel LA, ter Borg MN, Lindenbergh-Kortleve D, Jensen LT, Kooy- Winkelaar Y, Koning F, Boon L, Nieuwenhuis EE, Sollid LM, Fugger L, Samsom JN. Tolerance to ingested deamidated gliadin in mice is maintained by splenic, type 1 regulatory T cells. Gastroenterology. 2011; 141: e /j.gastro [PubMed: ] 60. Collado MC, Donat E, Ribes-Koninckx C, Calabuig M, Sanz Y. Imbalances in faecal and duodenal Bifidobacterium species composition in active and non-active coeliac disease. BMC Microbiol. 2008; 8: [pii] / [PubMed: ]
17 Marietta and Murray Page Collado MC, Donat E, Ribes-Koninckx C, Calabuig M, Sanz Y. Specific duodenal and faecal bacterial groups associated with paediatric coeliac disease. J Clin Pathol. 2009; 62: jcp [pii] /jcp [PubMed: ] 62. Kopecny J, Mrazek J, Fliegerova K, Fruhauf P, Tuckova L. The intestinal microflora of childhood patients with indicated celiac disease. Folia Microbiologica. 2008; 53: / s [PubMed: ] 63. Di Cagno R, De Angelis M, De Pasquale I, Ndagijimana M, Vernocchi P, Ricciuti P, Gagliardi F, Laghi L, Crecchio C, Guerzoni ME, Gobbetti M, Francavilla R. Duodenal and faecal microbiota of celiac children: molecular, phenotype and metabolome characterization. BMC Microbiol. 2011; 11: / [PubMed: ] 64. Hoorfar J, Buschard K, Dagnaes-Hansen F. Prophylactic nutritional modification of the incidence of diabetes in autoimmune non-obese diabetic (NOD) mice. Br J Nutr. 1993; 69: S [pii]. [PubMed: ] 65. Beales PE, Elliott RB, Flohe S, Hill JP, Kolb H, Pozzilli P, Wang GS, Wasmuth H, Scott FW. A multi-centre, blinded international trial of the effect of A(1) and A(2) beta-casein variants on diabetes incidence in two rodent models of spontaneous Type I diabetes. Diabetologia. 2002; 45: /s [PubMed: ] 66. Schmid S, Koczwara K, Schwinghammer S, Lampasona V, Ziegler AG, Bonifacio E. Delayed exposure to wheat and barley proteins reduces diabetes incidence in non-obese diabetic mice. Clin Immunol. 2004; 111: S X [pii] /j.clim [PubMed: ] 67. Funda DP, Kaas A, Bock T, Tlaskalova-Hogenova H, Buschard K. Gluten-free diet prevents diabetes in NOD mice. Diabetes Metab Res Rev. 1999; 15: [pii] /(SICI) (199909/10)15:5<323::AID-DMRR53>3.0.CO;2-P [PubMed: ] 68. Karges W, Hammond-McKibben D, Cheung RK, Visconti M, Shibuya N, Kemp D, Dosch HM. Immunological aspects of nutritional diabetes prevention in NOD mice: a pilot study for the cow s milk-based IDDM prevention trial. Diabetes. 1997; 46: [PubMed: ] 69. Hummel S, Ziegler AG. Early determinants of type 1 diabetes: experience from the BABYDIAB and BABYDIET studies. Am J Clin Nutr ajcn [pii] /ajcn Hummel S, Pfluger M, Hummel M, Bonifacio E, Ziegler AG. Primary dietary intervention study to reduce the risk of islet autoimmunity in children at increased risk for type 1 diabetes: the BABYDIET study. Diabetes Care. 2011; 34: dc [pii] /dc [PubMed: ] 71. Scott FW, Rowsell P, Wang GS, Burghardt K, Kolb H, Flohe S. Oral exposure to diabetespromoting food or immunomodulators in neonates alters gut cytokines and diabetes. Diabetes. 2002; 51: [PubMed: ] 72. Visser J, Hillebrands JL, Boer MW, Bos NA, Rozing J. Prevention of diabetes by a hydrolysed casein-based diet in diabetes-prone BioBreeding rats does not involve restoration of the defective natural regulatory T cell function. Diabetologia. 2009; 52: /s [PubMed: ] 73. Visser J, Brugman S, Klatter F, Vis L, Groen H, Strubbe J, Rozing J. Short-term dietary adjustment with a hydrolyzed casein-based diet postpones diabetes development in the diabetes-prone BB rat. Metabolism: clinical and experimental. 2003; 52: /meta [PubMed: ] 74. MacFarlane AJ, Burghardt KM, Kelly J, Simell T, Simell O, Altosaar I, Scott FW. A type 1 diabetes-related protein from wheat (Triticum aestivum). cdna clone of a wheat storage globulin, Glb1, linked to islet damage. J Biol Chem. 2003; 278: /jbc.M [PubMed: ] 75. Loit E, Melnyk CW, MacFarlane AJ, Scott FW, Altosaar I. Identification of three wheat globulin genes by screening a Triticum aestivum BAC genomic library with cdna from a diabetesassociated globulin. BMC plant biology. 2009; 9: / [PubMed: ] 76. Sapone A, de Magistris L, Pietzak M, Clemente MG, Tripathi A, Cucca F, Lampis R, Kryszak D, Carteni M, Generoso M, Iafusco D, Prisco F, Laghi F, Riegler G, Carratu R, Counts D, Fasano A.
18 Marietta and Murray Page 18 Zonulin upregulation is associated with increased gut permeability in subjects with type 1 diabetes and their relatives. Diabetes. 2006; 55: [PubMed: ] 77. Smecuol E, Sugai E, Niveloni S, Vazquez H, Pedreira S, Mazure R, Moreno ML, Label M, Maurino E, Fasano A, Meddings J, Bai JC. Permeability, zonulin production, and enteropathy in dermatitis herpetiformis. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association. 2005; 3: [PubMed: ] 78. Lammers KM, Lu R, Brownley J, Lu B, Gerard C, Thomas K, Rallabhandi P, Shea-Donohue T, Tamiz A, Alkan S, Netzel-Arnett S, Antalis T, Vogel SN, Fasano A. Gliadin induces an increase in intestinal permeability and zonulin release by binding to the chemokine receptor CXCR3. Gastroenterology. 2008; 135: e /j.gastro [PubMed: ] 79. Simpson M, Mojibian M, Barriga K, Scott FW, Fasano A, Rewers M, Norris JM. An exploration of Glo-3A antibody levels in children at increased risk for type 1 diabetes mellitus. Pediatr Diabetes. 2009; 10: /j x [PubMed: ] 80. Sonier B, Strom A, Wang GS, Patrick C, Crookshank JA, Mojibian M, MacFarlane AJ, Scott FW. Antibodies from a patient with type 1 diabetes and celiac disease bind to macrophages that express the scavenger receptor CD163. Canadian journal of gastroenterology = Journal canadien de gastroenterologie. 2011; 25: [PubMed: ] 81. Huibregtse IL, Marietta EV, Rashtak S, Koning F, Rottiers P, David CS, van Deventer SJ, Murray JA. Induction of antigen-specific tolerance by oral administration of Lactococcus lactis delivered immunodominant DQ8-restricted gliadin peptide in sensitized nonobese diabetic Abo Dq8 transgenic mice. J Immunol. 2009; 183: jimmunol [pii] /jimmunol [PubMed: ] 82. Steidler L, Hans W, Schotte L, Neirynck S, Obermeier F, Falk W, Fiers W, Remaut E. Treatment of murine colitis by Lactococcus lactis secreting interleukin-10. Science. 2000; 289: [PubMed: ] 83. Huibregtse IL, Snoeck V, de Creus A, Braat H, De Jong EC, Van Deventer SJ, Rottiers P. Induction of ovalbumin-specific tolerance by oral administration of Lactococcus lactis secreting ovalbumin. Gastroenterology. 2007; 133: /j.gastro [PubMed: ] 84. D Arienzo R, Maurano F, Luongo D, Mazzarella G, Stefanile R, Troncone R, Auricchio S, Ricca E, David C, Rossi M. Adjuvant effect of Lactobacillus casei in a mouse model of gluten sensitivity. Immunology letters. 2008; 119: /j.imlet [PubMed: ] 85. D Arienzo R, Stefanile R, Maurano F, Mazzarella G, Ricca E, Troncone R, Auricchio S, Rossi M. Immunomodulatory effects of Lactobacillus casei administration in a mouse model of gliadinsensitive enteropathy. Scandinavian journal of immunology. 2011; 74: /j x [PubMed: ] 86. Endo H, Higurashi T, Hosono K, Sakai E, Sekino Y, Iida H, Sakamoto Y, Koide T, Takahashi H, Yoneda M, Tokoro C, Inamori M, Abe Y, Nakajima A. Efficacy of Lactobacillus casei treatment on small bowel injury in chronic low-dose aspirin users: a pilot randomized controlled study. Journal of gastroenterology. 2011; 46: /s [PubMed: ] 87. Keech CL, Dromey J, Chen ZJ, Anderson RP, McCluskey J. Immune Tolerance Induced By Peptide Immunotherapy in An HLA Dq2-Dependent Mouse Model of Gluten Immunity. Gastroenterology. 2009; 136:A57 A Senger S, Luongo D, Maurano F, Mazzeo MF, Siciliano RA, Gianfrani C, David C, Troncone R, Auricchio S, Rossi M. Intranasal administration of a recombinant alpha-gliadin down-regulates the immune response to wheat gliadin in DQ8 transgenic mice. Immunology letters. 2003; 88: [PubMed: ] 89. Pinier M, Verdu EF, Nasser-Eddine M, David CS, Vezina A, Rivard N, Leroux JC. Polymeric binders suppress gliadin-induced toxicity in the intestinal epithelium. Gastroenterology. 2009; 136: /j.gastro [PubMed: ] 90. Malamut G, El Machhour R, Montcuquet N, Martin-Lanneree S, Dusanter-Fourt I, Verkarre V, Mention JJ, Rahmi G, Kiyono H, Butz EA, Brousse N, Cellier C, Cerf-Bensussan N, Meresse B. IL-15 triggers an antiapoptotic pathway in human intraepithelial lymphocytes that is a potential
19 Marietta and Murray Page 19 new target in celiac disease-associated inflammation and lymphomagenesis. J Clin Invest. 2010; 120: /JCI41344 [PubMed: ] 91. Drago S, El Asmar R, Di Pierro M, Grazia Clemente M, Tripathi A, Sapone A, Thakar M, Iacono G, Carroccio A, D Agate C, Not T, Zampini L, Catassi C, Fasano A. Gliadin, zonulin and gut permeability: Effects on celiac and non-celiac intestinal mucosa and intestinal cell lines. Scand J Gastroenterol. 2006; 41: / [PubMed: ] 92. Gopalakrishnan S, Tripathi A, Tamiz AP, Alkan SS, Pandey NB. Larazotide acetate promotes tight junction assembly in epithelial cells. Peptides /j.peptides Gopalakrishnan S, Durai M, Kitchens K, Tamiz AP, Somerville R, Ginski M, Paterson BM, Murray JA, Verdu EF, Alkan SS, Pandey NB. Larazotide acetate regulates epithelial tight junctions in vitro and in vivo. Peptides /j.peptides Bergamo P, Maurano F, Mazzarella G, Iaquinto G, Vocca I, Rivelli AR, De Falco E, Gianfrani C, Rossi M. Immunological evaluation of the alcohol-soluble protein fraction from gluten-free grains in relation to celiac disease. Molecular nutrition & food research. 2011; 55: / mnfr [PubMed: ]
20 Marietta and Murray Page 20 Figure 1. Pathogenic Steps of Celiac Disease That Each Animal Model Species Can Address Depicted in the illustration is each of the seven main pathogenic steps of celiac disease, and their descriptions are listed in the left column of the corresponding table. Listed in the right column are the species of the animal models that mimic the steps.
21 Marietta and Murray Page 21 Figure 2. Specific Animal Models Used to Test Novel Therapies for Celiac Disease Depicted in the illustration is each of the seven main pathogenic steps of celiac disease. Listed below in the table are the novel therapies generated for targeting specific steps and the species (animal models) that have been used to test the therapies. References are provided in the far right column.
Diseases of the gastrointestinal system Dr H Awad Lecture 5: diseases of the small intestine
 Diseases of the gastrointestinal system 2018 Dr H Awad Lecture 5: diseases of the small intestine Small intestinal villi Small intestinal villi -Villi are tall, finger like mucosal projections, found
Diseases of the gastrointestinal system 2018 Dr H Awad Lecture 5: diseases of the small intestine Small intestinal villi Small intestinal villi -Villi are tall, finger like mucosal projections, found
Diagnostic Testing Algorithms for Celiac Disease
 Diagnostic Testing Algorithms for Celiac Disease HOT TOPIC / 2018 Presenter: Melissa R. Snyder, Ph.D. Co-Director, Antibody Immunology Laboratory Department of Laboratory Medicine and Pathology, Mayo Clinic
Diagnostic Testing Algorithms for Celiac Disease HOT TOPIC / 2018 Presenter: Melissa R. Snyder, Ph.D. Co-Director, Antibody Immunology Laboratory Department of Laboratory Medicine and Pathology, Mayo Clinic
Celiac Disease: The Quintessential Autoimmune Disease Ivor D. Hill, MB, ChB, MD.
 Celiac Disease: The Quintessential Autoimmune Disease Ivor D. Hill, MB, ChB, MD..... Celiac Disease Autoimmune Diseases What are they? How do you get them? Why does it matter? Celiac Disease Autoimmune
Celiac Disease: The Quintessential Autoimmune Disease Ivor D. Hill, MB, ChB, MD..... Celiac Disease Autoimmune Diseases What are they? How do you get them? Why does it matter? Celiac Disease Autoimmune
Primary Care Update January 26 & 27, 2017 Celiac Disease: Concepts & Conundrums
 Primary Care Update January 26 & 27, 2017 Celiac Disease: Concepts & Conundrums Alia Hasham, MD Assistant Professor Division of Gastroenterology, Hepatology & Nutrition What is the Preferred Initial Test
Primary Care Update January 26 & 27, 2017 Celiac Disease: Concepts & Conundrums Alia Hasham, MD Assistant Professor Division of Gastroenterology, Hepatology & Nutrition What is the Preferred Initial Test
Baboons Affected by Hereditary Chronic Diarrhea as a Possible Non-Human Primate Model of Celiac Disease
 Baboons Affected by Hereditary Chronic Diarrhea as a Possible Non-Human Primate Model of Celiac Disease Debby Kryszak 1, Henry McGill 2, Michelle Leland 2,, Alessio Fasano 1 1. Center for Celiac Research,
Baboons Affected by Hereditary Chronic Diarrhea as a Possible Non-Human Primate Model of Celiac Disease Debby Kryszak 1, Henry McGill 2, Michelle Leland 2,, Alessio Fasano 1 1. Center for Celiac Research,
November Laboratory Testing for Celiac Disease. Inflammation in Celiac Disease
 November 2011 Gary Copland, MD Chair, Department of Pathology, Unity Hospital Laboratory Medical Director, AMC Crossroads Chaska and AMC Crossroads Dean Lakes Laboratory Testing for Celiac Disease Celiac
November 2011 Gary Copland, MD Chair, Department of Pathology, Unity Hospital Laboratory Medical Director, AMC Crossroads Chaska and AMC Crossroads Dean Lakes Laboratory Testing for Celiac Disease Celiac
Disclosures GLUTEN RELATED DISORDERS CELIAC DISEASE UPDATE OR GLUTEN RELATED DISORDERS 6/9/2015
 Disclosures CELIAC DISEASE UPDATE OR GLUTEN RELATED DISORDERS 2015 Scientific Advisory Board: Alvine Pharmaceuticals, Alba Therapeutics, ImmunsanT Peter HR Green MD Columbia University New York, NY GLUTEN
Disclosures CELIAC DISEASE UPDATE OR GLUTEN RELATED DISORDERS 2015 Scientific Advisory Board: Alvine Pharmaceuticals, Alba Therapeutics, ImmunsanT Peter HR Green MD Columbia University New York, NY GLUTEN
Activation of Innate and not Adaptive Immune system in Gluten Sensitivity
 Activation of Innate and not Adaptive Immune system in Gluten Sensitivity Update: Differential mucosal IL-17 expression in gluten sensitivity and the autoimmune enteropathy celiac disease A. Sapone, L.
Activation of Innate and not Adaptive Immune system in Gluten Sensitivity Update: Differential mucosal IL-17 expression in gluten sensitivity and the autoimmune enteropathy celiac disease A. Sapone, L.
Diagnosis Diagnostic principles Confirm diagnosis before treating
 Diagnosis 1 1 Diagnosis Diagnostic principles Confirm diagnosis before treating Diagnosis of Celiac Disease mandates a strict gluten-free diet for life following the diet is not easy QOL implications Failure
Diagnosis 1 1 Diagnosis Diagnostic principles Confirm diagnosis before treating Diagnosis of Celiac Disease mandates a strict gluten-free diet for life following the diet is not easy QOL implications Failure
See Policy CPT CODE section below for any prior authorization requirements
 Effective Date: 1/1/2019 Section: LAB Policy No: 404 Medical Policy Committee Approved Date: 12/17; 12/18 1/1/19 Medical Officer Date APPLIES TO: All lines of business See Policy CPT CODE section below
Effective Date: 1/1/2019 Section: LAB Policy No: 404 Medical Policy Committee Approved Date: 12/17; 12/18 1/1/19 Medical Officer Date APPLIES TO: All lines of business See Policy CPT CODE section below
Is It Celiac Disease or Gluten Sensitivity?
 Is It Celiac Disease or Gluten Sensitivity? Mark T. DeMeo MD, FACG Rush University Med Center Case Study 35 y/o female Complains of diarrhea, bloating, arthralgias, and foggy mentation Cousin with celiac
Is It Celiac Disease or Gluten Sensitivity? Mark T. DeMeo MD, FACG Rush University Med Center Case Study 35 y/o female Complains of diarrhea, bloating, arthralgias, and foggy mentation Cousin with celiac
Larazotide Acetate. Alessio Fasano, M.D. Mucosal Biology Research Center and Center for Celiac Research University of Maryland School of Medicine
 Larazotide Acetate Alessio Fasano, M.D. Mucosal Biology Research Center and Center for Celiac Research University of Maryland School of Medicine Alternative/Integrative Approaches To The Gluten Free Diet
Larazotide Acetate Alessio Fasano, M.D. Mucosal Biology Research Center and Center for Celiac Research University of Maryland School of Medicine Alternative/Integrative Approaches To The Gluten Free Diet
Diet Isn t Working, We Need to Do Something Else
 Diet Isn t Working, We Need to Do Something Else Ciarán P Kelly, MD Celiac Center Beth Israel Deaconess Medical Center & Celiac Program Harvard Medical School Boston Gluten Free Diet (GFD) Very good but
Diet Isn t Working, We Need to Do Something Else Ciarán P Kelly, MD Celiac Center Beth Israel Deaconess Medical Center & Celiac Program Harvard Medical School Boston Gluten Free Diet (GFD) Very good but
Celiac disease: Beyond Glutenfree. AmerEl Sayed, MD LSGE- Annual Meeting 2014
 Celiac disease: Beyond Glutenfree diet AmerEl Sayed, MD LSGE- Annual Meeting 2014 Pathogenesis Auto-immune disease, 1% western population 3 main pathways Host Genetic background HLA-DQ2 HLA-DQ8 Non-HLA
Celiac disease: Beyond Glutenfree diet AmerEl Sayed, MD LSGE- Annual Meeting 2014 Pathogenesis Auto-immune disease, 1% western population 3 main pathways Host Genetic background HLA-DQ2 HLA-DQ8 Non-HLA
Gluten Sensitivity Fact from Myth. Disclosures OBJECTIVES 18/09/2013. Justine Turner MD PhD University of Alberta. None Relevant
 Gluten Sensitivity Fact from Myth Justine Turner MD PhD University of Alberta Disclosures None Relevant OBJECTIVES Understand the spectrum of gluten disorders Develop a diagnostic algorithm for gluten
Gluten Sensitivity Fact from Myth Justine Turner MD PhD University of Alberta Disclosures None Relevant OBJECTIVES Understand the spectrum of gluten disorders Develop a diagnostic algorithm for gluten
Celiac Disease Ce. Celiac Disease. Barry Z. Hirsch, M.D. Baystate Pediatric Gastroenterology and Nutrition. baystatehealth.org/bch
 Celiac Disease Ce Celiac Disease Barry Z. Hirsch, M.D. Baystate Pediatric Gastroenterology and Nutrition baystatehealth.org/bch Autoimmune Disease Inappropriate inflammation 1 1/21/15 Celiac Disease Classic
Celiac Disease Ce Celiac Disease Barry Z. Hirsch, M.D. Baystate Pediatric Gastroenterology and Nutrition baystatehealth.org/bch Autoimmune Disease Inappropriate inflammation 1 1/21/15 Celiac Disease Classic
Challenges in Celiac Disease. Adam Stein, MD Director of Nutrition Support Northwestern University Feinberg School of Medicine
 Challenges in Celiac Disease Adam Stein, MD Director of Nutrition Support Northwestern University Feinberg School of Medicine Disclosures None Overview Celiac disease Cases Celiac disease Inappropriate
Challenges in Celiac Disease Adam Stein, MD Director of Nutrition Support Northwestern University Feinberg School of Medicine Disclosures None Overview Celiac disease Cases Celiac disease Inappropriate
Therapeutical implication of regulatory cells and cytokines in celiac disease
 Institute of Food Sciences, CNR Avellino, Italy Therapeutical implication of regulatory cells and cytokines in celiac disease Carmen Gianfrani Mastering the coeliac condition: from medicine to social sciences
Institute of Food Sciences, CNR Avellino, Italy Therapeutical implication of regulatory cells and cytokines in celiac disease Carmen Gianfrani Mastering the coeliac condition: from medicine to social sciences
New Insights on Gluten Sensitivity
 New Insights on Gluten Sensitivity Sheila E. Crowe, MD, FRCPC, FACP, FACG, AGAF Department of Medicine University of California, San Diego Page 1 1 low fat diet low carb diet gluten free diet low fat diet
New Insights on Gluten Sensitivity Sheila E. Crowe, MD, FRCPC, FACP, FACG, AGAF Department of Medicine University of California, San Diego Page 1 1 low fat diet low carb diet gluten free diet low fat diet
Celiac disease Crohn s disease Ulcerative colitis Pseudomembranous colitis
 2017 / 2018 2nd semester/3rd practice Celiac disease Crohn s disease Ulcerative colitis Pseudomembranous colitis Semmelweis University 2nd Department of Pathology CELIAC DISEASE = Gluten-sensitive enteropathy
2017 / 2018 2nd semester/3rd practice Celiac disease Crohn s disease Ulcerative colitis Pseudomembranous colitis Semmelweis University 2nd Department of Pathology CELIAC DISEASE = Gluten-sensitive enteropathy
Meredythe A. McNally, M.D. Gastroenterology Associates of Cleveland Beachwood, OH
 Meredythe A. McNally, M.D. Gastroenterology Associates of Cleveland Beachwood, OH Case in point 42 year old woman with bloating, gas, intermittent diarrhea alternating with constipation, told she has IBS
Meredythe A. McNally, M.D. Gastroenterology Associates of Cleveland Beachwood, OH Case in point 42 year old woman with bloating, gas, intermittent diarrhea alternating with constipation, told she has IBS
Celiac Disease. Etiology. Food Intolerance:Celiac Disease and Gluten Sensitivity-A Guide for Healthy Lifestyles
 Food Intolerance:Celiac Disease and Gluten Sensitivity-A Guide for Healthy Lifestyles Ellen Karlin 2017 Celiac Disease World s most common genetic food disorder Rising prevalence - over past 5 decades,
Food Intolerance:Celiac Disease and Gluten Sensitivity-A Guide for Healthy Lifestyles Ellen Karlin 2017 Celiac Disease World s most common genetic food disorder Rising prevalence - over past 5 decades,
CELIAC DISEASE - GENERAL AND LABORATORY ASPECTS Prof. Xavier Bossuyt, Ph.D. Laboratory Medicine, Immunology, University Hospital Leuven, Belgium
 CELIAC DISEASE - GENERAL AND LABORATORY ASPECTS Prof. Xavier Bossuyt, Ph.D. Laboratory Medicine, Immunology, University Hospital Leuven, Belgium 5.1 Introduction Celiac disease is a chronic immune-mediated
CELIAC DISEASE - GENERAL AND LABORATORY ASPECTS Prof. Xavier Bossuyt, Ph.D. Laboratory Medicine, Immunology, University Hospital Leuven, Belgium 5.1 Introduction Celiac disease is a chronic immune-mediated
CONTEMPORARY CONCEPT ON BASIC APSECTS OF GLUTEN-SENSITIVE ENTEROPATHY IN ELDERLY PATIENTS
 VIII, 2014, 1 33. 1,. 2,. - 1,. 1. 3 1,., 2,., 3, CONTEMPORARY CONCEPT ON BASIC APSECTS OF GLUTEN-SENSITIVE ENTEROPATHY IN ELDERLY PATIENTS Ts. Velikova 1, Z. Spassova 2,. Ivanova-Todorova 1, D. Kyurkchiev
VIII, 2014, 1 33. 1,. 2,. - 1,. 1. 3 1,., 2,., 3, CONTEMPORARY CONCEPT ON BASIC APSECTS OF GLUTEN-SENSITIVE ENTEROPATHY IN ELDERLY PATIENTS Ts. Velikova 1, Z. Spassova 2,. Ivanova-Todorova 1, D. Kyurkchiev
Spectrum of Gluten Disorders
 Food Intolerance:Celiac Disease and Gluten Sensitivity-A Guide for Healthy Lifestyles Ellen Karlin 2018 Spectrum of Gluten Disorders Wheat allergy - prevalence 3-8 % (up to 3 years old) Non-celiac gluten
Food Intolerance:Celiac Disease and Gluten Sensitivity-A Guide for Healthy Lifestyles Ellen Karlin 2018 Spectrum of Gluten Disorders Wheat allergy - prevalence 3-8 % (up to 3 years old) Non-celiac gluten
Peter HR Green MD. Columbia University New York, NY
 CELIAC DISEASE, 2008 Peter HR Green MD Celiac Disease Center Columbia University New York, NY pg11@columbia.edu DIAGNOSIS OF CELIAC DISEASE Presence of consistent pathology and response to a gluten-free
CELIAC DISEASE, 2008 Peter HR Green MD Celiac Disease Center Columbia University New York, NY pg11@columbia.edu DIAGNOSIS OF CELIAC DISEASE Presence of consistent pathology and response to a gluten-free
Functional Medicine Is the application of alternative holistic measures to show people how to reverse thyroid conditions, endocrine issues, hormone
 Functional Medicine Is the application of alternative holistic measures to show people how to reverse thyroid conditions, endocrine issues, hormone issues, fibromyalgia, autoimmunity diseases and the like.
Functional Medicine Is the application of alternative holistic measures to show people how to reverse thyroid conditions, endocrine issues, hormone issues, fibromyalgia, autoimmunity diseases and the like.
Health Canada s Position on Gluten-Free Claims
 June 2012 Bureau of Chemical Safety, Food Directorate, Health Products and Food Branch 0 Table of Contents Background... 2 Regulatory Requirements for Gluten-Free Foods... 2 Recent advances in the knowledge
June 2012 Bureau of Chemical Safety, Food Directorate, Health Products and Food Branch 0 Table of Contents Background... 2 Regulatory Requirements for Gluten-Free Foods... 2 Recent advances in the knowledge
Celiac Disease For Dummies By Sheila Crowe, Ian Blumer READ ONLINE
 Celiac Disease For Dummies By Sheila Crowe, Ian Blumer READ ONLINE Celiac disease definition, a hereditary digestive disorder involving intolerance to gluten, usually occurring in young children, characterized
Celiac Disease For Dummies By Sheila Crowe, Ian Blumer READ ONLINE Celiac disease definition, a hereditary digestive disorder involving intolerance to gluten, usually occurring in young children, characterized
Celiac & Gluten Sensitivity; serum
 TEST NAME: Celiac & Gluten Sensitivity (Serum) Celiac & Gluten Sensitivity; serum ANTIBODIES REFERENCE RESULT/UNIT INTERVAL NEG WEAK POS POSITIVE Tissue Transglutaminase (ttg) IgA 1420 U < 20.0 Tissue
TEST NAME: Celiac & Gluten Sensitivity (Serum) Celiac & Gluten Sensitivity; serum ANTIBODIES REFERENCE RESULT/UNIT INTERVAL NEG WEAK POS POSITIVE Tissue Transglutaminase (ttg) IgA 1420 U < 20.0 Tissue
EAT ACCORDING TO YOUR GENES. NGx-Gluten TM. Personalized Nutrition Report
 EAT ACCORDING TO YOUR GENES NGx-Gluten TM Personalized Nutrition Report Introduction Hello Caroline: Nutrigenomix is pleased to provide you with your NGx-Gluten TM Personalized Nutrition Report based on
EAT ACCORDING TO YOUR GENES NGx-Gluten TM Personalized Nutrition Report Introduction Hello Caroline: Nutrigenomix is pleased to provide you with your NGx-Gluten TM Personalized Nutrition Report based on
DEAMIDATED GLIADIN PEPTIDES IN COELIAC DISEASE DIAGNOSTICS
 DEAMIDATED GLIADIN PEPTIDES IN COELIAC DISEASE DIAGNOSTICS Z. Vanickova 1, P. Kocna 1, K. Topinkova 1, M. Dvorak 2 1 Institute of Clinical Biochemistry & Laboratory Diagnostics; 2 4th Medical Department,
DEAMIDATED GLIADIN PEPTIDES IN COELIAC DISEASE DIAGNOSTICS Z. Vanickova 1, P. Kocna 1, K. Topinkova 1, M. Dvorak 2 1 Institute of Clinical Biochemistry & Laboratory Diagnostics; 2 4th Medical Department,
BIOPSY AVOIDANCE IN CHILDREN: THE EVIDENCE
 BIOPSY AVOIDANCE IN CHILDREN: THE EVIDENCE Steffen Husby Hans Christian Andersen Children s Hospital Odense University Hospital DK-5000 Odense C, Denmark Agenda Background Algorithm Symptoms HLA Antibodies
BIOPSY AVOIDANCE IN CHILDREN: THE EVIDENCE Steffen Husby Hans Christian Andersen Children s Hospital Odense University Hospital DK-5000 Odense C, Denmark Agenda Background Algorithm Symptoms HLA Antibodies
Immune mediated enteropathies. Aurora Tatu Bern 26/07/2017
 Immune mediated enteropathies Aurora Tatu Bern 26/07/2017 Definition/classification Systemic disease, mediated by antibodies, caracterised by histological changes of the small bowel Coeliac and noncoeliac
Immune mediated enteropathies Aurora Tatu Bern 26/07/2017 Definition/classification Systemic disease, mediated by antibodies, caracterised by histological changes of the small bowel Coeliac and noncoeliac
Coeliac disease: pathogenesis. Riccardo Troncone
 Coeliac disease: pathogenesis Riccardo Troncone Department of Pediatrics & European Laboratory for the Investigation of Food-Induced Diseases University Federico II, Naples, Italy Definition of Celiac
Coeliac disease: pathogenesis Riccardo Troncone Department of Pediatrics & European Laboratory for the Investigation of Food-Induced Diseases University Federico II, Naples, Italy Definition of Celiac
Evidence Based Guideline
 Evidence Based Guideline Serologic Diagnosis of Celiac Disease File Name: Origination: Last CAP Review: Next CAP Review: Last Review: serologic_diagnosis_of_celiac_disease 4/2012 Description of Procedure
Evidence Based Guideline Serologic Diagnosis of Celiac Disease File Name: Origination: Last CAP Review: Next CAP Review: Last Review: serologic_diagnosis_of_celiac_disease 4/2012 Description of Procedure
Gluten sensitivity in Multiple Sclerosis Experimental myth or clinical truth?
 Gluten sensitivity in Multiple Sclerosis Experimental myth or clinical truth? Annals of the New York Academy of Sciences, Vol 1173, Issue 1, page 44, Issue published online 3 Sep 2009. Dana Ben-Ami Shor,
Gluten sensitivity in Multiple Sclerosis Experimental myth or clinical truth? Annals of the New York Academy of Sciences, Vol 1173, Issue 1, page 44, Issue published online 3 Sep 2009. Dana Ben-Ami Shor,
Coeliac disease. Do I have coeliac. disease? Diagnosis, monitoring & susceptibilty. Laboratory flowsheet included
 Laboratory flowsheet included I have coeliac disease. What monitoring tests should be performed? Do I have coeliac disease? Are either of our children susceptible to coeliac disease? Monitoring tests Diagnostic
Laboratory flowsheet included I have coeliac disease. What monitoring tests should be performed? Do I have coeliac disease? Are either of our children susceptible to coeliac disease? Monitoring tests Diagnostic
Celiac Disease. Detlef Schuppan HARVARD MEDICAL SCHOOL
 Celiac Disease Detlef Schuppan Falk Symposium in the Intestinal Tract: Pathogenesis and Treatment, Kiev,, Ukraine, May 15-16, 16, 2009 HARVARD MEDICAL SCHOOL Celiac Disease Intolerance to gluten from wheat,
Celiac Disease Detlef Schuppan Falk Symposium in the Intestinal Tract: Pathogenesis and Treatment, Kiev,, Ukraine, May 15-16, 16, 2009 HARVARD MEDICAL SCHOOL Celiac Disease Intolerance to gluten from wheat,
Celiac Disease: The Future. Alessio Fasano, M.D. Mucosal Biology Research Center University of Maryland School of Medicine
 Celiac Disease: The Future Alessio Fasano, M.D. Mucosal Biology Research Center University of Maryland School of Medicine Normal small bowel Celiac disease Gluten Gluten-free diet Treatment Only treatment
Celiac Disease: The Future Alessio Fasano, M.D. Mucosal Biology Research Center University of Maryland School of Medicine Normal small bowel Celiac disease Gluten Gluten-free diet Treatment Only treatment
Current Management of Celiac Disease and Identifying an Appropriate Patient Population(s) for Pharmacologic Therapies in Adult Patients
 Current Management of Celiac Disease and Identifying an Appropriate Patient Population(s) for Pharmacologic Therapies in Adult Patients Joe Murray The Mayo Clinic 1 DISCLOSURES Relevant Financial Relationship(s)
Current Management of Celiac Disease and Identifying an Appropriate Patient Population(s) for Pharmacologic Therapies in Adult Patients Joe Murray The Mayo Clinic 1 DISCLOSURES Relevant Financial Relationship(s)
Epidemiology. The old Celiac Disease Epidemiology:
 Epidemiology 1 1 Epidemiology The old Celiac Disease Epidemiology: A rare disorder typical of infancy Wide incidence fluctuates in space (1/400 Ireland to 1/10000 Denmark) and in time A disease of essentially
Epidemiology 1 1 Epidemiology The old Celiac Disease Epidemiology: A rare disorder typical of infancy Wide incidence fluctuates in space (1/400 Ireland to 1/10000 Denmark) and in time A disease of essentially
ImuPro shows you the way to the right food for you. And your path for better health.
 Your personal ImuPro Screen + documents Sample ID: 33333 Dear, With this letter, you will receive the ImuPro result for your personal IgG food allergy test. This laboratory report contains your results
Your personal ImuPro Screen + documents Sample ID: 33333 Dear, With this letter, you will receive the ImuPro result for your personal IgG food allergy test. This laboratory report contains your results
Am I a Silly Yak? Laura Zakowski, MD. No financial disclosures
 Am I a Silly Yak? Laura Zakowski, MD No financial disclosures Patient NP 21 year old male with chronic headaches for 6 years extensively evaluated and treated Acupuncturist suggests testing for celiac
Am I a Silly Yak? Laura Zakowski, MD No financial disclosures Patient NP 21 year old male with chronic headaches for 6 years extensively evaluated and treated Acupuncturist suggests testing for celiac
CELIAC SPRUE. What Happens With Celiac Disease
 CELIAC SPRUE Celiac Disease (CD) is a lifelong, digestive disorder affecting children and adults. When people with CD eat foods that contain gluten, it creates an immune-mediated toxic reaction that causes
CELIAC SPRUE Celiac Disease (CD) is a lifelong, digestive disorder affecting children and adults. When people with CD eat foods that contain gluten, it creates an immune-mediated toxic reaction that causes
Food Intolerance & Expertise SARAH KEOGH CONSULTANT DIETITIAN EATWELL FOOD & NUTRITION
 Food Intolerance & Expertise SARAH KEOGH CONSULTANT DIETITIAN EATWELL FOOD & NUTRITION Food Intolerance & Expertise What is food intolerance? Common food intolerances Why are consumers claiming more food
Food Intolerance & Expertise SARAH KEOGH CONSULTANT DIETITIAN EATWELL FOOD & NUTRITION Food Intolerance & Expertise What is food intolerance? Common food intolerances Why are consumers claiming more food
Celiac Disease 1/13/2016. Objectives. Question 1. Understand the plethora of conditions or symptoms that require testing for Celiac Disease (CD)
 Celiac Disease MONTE E. TROUTMAN, DO, FACOI JANUARY 6, 2016 Objectives Understand the plethora of conditions or symptoms that require testing for Celiac Disease (CD) Develop a knowledge of testing needed
Celiac Disease MONTE E. TROUTMAN, DO, FACOI JANUARY 6, 2016 Objectives Understand the plethora of conditions or symptoms that require testing for Celiac Disease (CD) Develop a knowledge of testing needed
Sheila E. Crowe, MD, FACG
 1A: Upper Gut Celiac Disease: When to Look and How? Sheila E. Crowe, MD, FACG Learning Objectives At the end of this presentation, the successful learner should be able to: Identify the many groups of
1A: Upper Gut Celiac Disease: When to Look and How? Sheila E. Crowe, MD, FACG Learning Objectives At the end of this presentation, the successful learner should be able to: Identify the many groups of
'Every time I eat dairy foods I become ill, could I have a milk allergy.? '. Factors involved in the development of cow's milk allergy:
 'Every time I eat dairy foods I become ill, could I have a milk allergy.? '. Dairy allergy is relatively common in the community. The unpleasant symptoms some people experience after eating dairy foods
'Every time I eat dairy foods I become ill, could I have a milk allergy.? '. Dairy allergy is relatively common in the community. The unpleasant symptoms some people experience after eating dairy foods
Gluten-Free China Gastro Q&A
 Gluten-Free China Gastro Q&A Akiko Natalie Tomonari MD akiko.tomonari@parkway.cn Gastroenterology Specialist ParkwayHealth Introduction (of myself) Born in Japan, Raised in Maryland, USA Graduated from
Gluten-Free China Gastro Q&A Akiko Natalie Tomonari MD akiko.tomonari@parkway.cn Gastroenterology Specialist ParkwayHealth Introduction (of myself) Born in Japan, Raised in Maryland, USA Graduated from
No relevant financial relationships to disclose
 CELIAC DISEASE Michael H. Piper, MD, FACP, FACG Gastroenterology Program Director Chief of Gastroenterology Providence-Providence Park Hospitals/St. John Macomb Hospital No relevant financial relationships
CELIAC DISEASE Michael H. Piper, MD, FACP, FACG Gastroenterology Program Director Chief of Gastroenterology Providence-Providence Park Hospitals/St. John Macomb Hospital No relevant financial relationships
Pediatric Food Allergies: Physician and Parent. Robert Anderson MD Rachel Anderson Syracuse, NY March 3, 2018
 Pediatric Food Allergies: Physician and Parent Robert Anderson MD Rachel Anderson Syracuse, NY March 3, 2018 Learning Objectives Identify risk factors for food allergies Identify clinical manifestations
Pediatric Food Allergies: Physician and Parent Robert Anderson MD Rachel Anderson Syracuse, NY March 3, 2018 Learning Objectives Identify risk factors for food allergies Identify clinical manifestations
The lab is open, the tests are available. Read on for much more information.
 From: *Dr. Tom O'Bryan * thedr.com Subject: The Tests That We've Been Waiting For ~ Gluten Sensitivity Related Testing Reply: karen@thedr.com Having trouble viewing this email? Click
From: *Dr. Tom O'Bryan * thedr.com Subject: The Tests That We've Been Waiting For ~ Gluten Sensitivity Related Testing Reply: karen@thedr.com Having trouble viewing this email? Click
Food Allergies on the Rise in American Children
 Transcript Details This is a transcript of an educational program accessible on the ReachMD network. Details about the program and additional media formats for the program are accessible by visiting: https://reachmd.com/programs/hot-topics-in-allergy/food-allergies-on-the-rise-in-americanchildren/3832/
Transcript Details This is a transcript of an educational program accessible on the ReachMD network. Details about the program and additional media formats for the program are accessible by visiting: https://reachmd.com/programs/hot-topics-in-allergy/food-allergies-on-the-rise-in-americanchildren/3832/
Food Allergies: Fact from Fiction
 Transcript Details This is a transcript of an educational program accessible on the ReachMD network. Details about the program and additional media formats for the program are accessible by visiting: https://reachmd.com/programs/gi-insights/food-allergies-fact-from-fiction/3598/
Transcript Details This is a transcript of an educational program accessible on the ReachMD network. Details about the program and additional media formats for the program are accessible by visiting: https://reachmd.com/programs/gi-insights/food-allergies-fact-from-fiction/3598/
Celiac Disease and Non Celiac Gluten Sensitivity. John R Cangemi, MD Mayo Clinic Florida
 Celiac Disease and Non Celiac Gluten Sensitivity John R Cangemi, MD Mayo Clinic Florida DISCLOSURE Commercial Interest None Off Label Usage None Learning Objectives Review the clinical presentation of
Celiac Disease and Non Celiac Gluten Sensitivity John R Cangemi, MD Mayo Clinic Florida DISCLOSURE Commercial Interest None Off Label Usage None Learning Objectives Review the clinical presentation of
588-Complete Dietary Antigen Testing
 REPORT-1857 9 Dunwoody Park, Suite 121 Dunwoody, GA 3338 P: 678-736-6374 F: 77-674-171 Email: info@dunwoodylabs.com www.dunwoodylabs.com PATIENT INFO NAME: SAMPE PATIENT REQUISITION ID: 1857 SAMPE ID:
REPORT-1857 9 Dunwoody Park, Suite 121 Dunwoody, GA 3338 P: 678-736-6374 F: 77-674-171 Email: info@dunwoodylabs.com www.dunwoodylabs.com PATIENT INFO NAME: SAMPE PATIENT REQUISITION ID: 1857 SAMPE ID:
GUIDANCE ON THE DIAGNOSIS AND MANAGEMENT OF LACTOSE INTOLERANCE
 GUIDANCE ON THE DIAGNOSIS AND MANAGEMENT OF LACTOSE INTOLERANCE These are the lactose intolerance guidelines and it is recommended that they are used in conjunction with the Cow s Milk Allergy guidance.
GUIDANCE ON THE DIAGNOSIS AND MANAGEMENT OF LACTOSE INTOLERANCE These are the lactose intolerance guidelines and it is recommended that they are used in conjunction with the Cow s Milk Allergy guidance.
FOXP3 EXPRESSION IN HUMAN CANCER CELLS
 FOXP3 EXPRESSION IN HUMAN CANCER CELLS Vaios Karanikas EU Marie Curie Fellow Cancer Immunology Unit Department of Immunology and Histocompatibility School of Medicine University of Thessaly Larissa, Greece
FOXP3 EXPRESSION IN HUMAN CANCER CELLS Vaios Karanikas EU Marie Curie Fellow Cancer Immunology Unit Department of Immunology and Histocompatibility School of Medicine University of Thessaly Larissa, Greece
Seriously, CELIAC. talk.
 Seriously, Celiac Disease. talk. If you have celiac disease, your family members might have it too. Talk to them about your experience and how celiac disease runs in families. Tell them the facts. Urge
Seriously, Celiac Disease. talk. If you have celiac disease, your family members might have it too. Talk to them about your experience and how celiac disease runs in families. Tell them the facts. Urge
DR.RAJIV SHARMA BOOK SERIES 2
 DR.RAJIV SHARMA BOOK SERIES 2 CELIAC DISEASE AND GLUTEN 1 DR.RAJIV SHARMA CELIAC DISEASE AND GLUTEN GLUTEN IS LIKE AIR. ITS EVERYWHERE. As long as you have a beating heart you cannot avoid Gluten. Gluten
DR.RAJIV SHARMA BOOK SERIES 2 CELIAC DISEASE AND GLUTEN 1 DR.RAJIV SHARMA CELIAC DISEASE AND GLUTEN GLUTEN IS LIKE AIR. ITS EVERYWHERE. As long as you have a beating heart you cannot avoid Gluten. Gluten
Sequoia Education Systems, Inc. 1
 Functional Medicine University s Functional Diagnostic Medicine Program Module 3 * FDMT 527C The Elimination Diet & The Modified Elimination Diet Wayne L. Sodano, D.C., D.A.B.C.I. & Ron Grisanti, D.C.,
Functional Medicine University s Functional Diagnostic Medicine Program Module 3 * FDMT 527C The Elimination Diet & The Modified Elimination Diet Wayne L. Sodano, D.C., D.A.B.C.I. & Ron Grisanti, D.C.,
GI Allergy and Tolerance. Jon A. Vanderhoof, M.D. Division of Gastroenterology/Nutrition Boston Children s Hospital Harvard Medical School
 GI Allergy and Tolerance Jon A. Vanderhoof, M.D. Division of Gastroenterology/Nutrition Boston Children s Hospital Harvard Medical School Disclosure Medical Advisor- Mead Johnson Nutrition Food Allergy
GI Allergy and Tolerance Jon A. Vanderhoof, M.D. Division of Gastroenterology/Nutrition Boston Children s Hospital Harvard Medical School Disclosure Medical Advisor- Mead Johnson Nutrition Food Allergy
Living with Coeliac Disease Information & Support is key
 Living with Coeliac Disease Information & Support is key Mary Twohig Chairperson Coeliac Society of Ireland What is Coeliac Disease? LIVING WITH COELIAC DISEASE Fact Not Fad Auto immune disease - the body
Living with Coeliac Disease Information & Support is key Mary Twohig Chairperson Coeliac Society of Ireland What is Coeliac Disease? LIVING WITH COELIAC DISEASE Fact Not Fad Auto immune disease - the body
HL-A8: a genetic link between dermatitis herpetiformis and gluten-sensitive enteropathy
 HL-A8: a genetic link between dermatitis herpetiformis and gluten-sensitive enteropathy Stephen I. Katz,, G. Nicholas Rogentine, Warren Strober J Clin Invest. 1972;51(11):2977-2980. https://doi.org/10.1172/jci107123.
HL-A8: a genetic link between dermatitis herpetiformis and gluten-sensitive enteropathy Stephen I. Katz,, G. Nicholas Rogentine, Warren Strober J Clin Invest. 1972;51(11):2977-2980. https://doi.org/10.1172/jci107123.
DDW WRAP-UP 2012 CELIAC DISEASE. Anju Sidhu MD University of Louisville Gastroenterology, Hepatology and Nutrition June 21, 2012
 DDW WRAP-UP 2012 CELIAC DISEASE Anju Sidhu MD University of Louisville Gastroenterology, Hepatology and Nutrition June 21, 2012 OVERVIEW Definition Susceptibility The Changing Clinical Presentation Medical
DDW WRAP-UP 2012 CELIAC DISEASE Anju Sidhu MD University of Louisville Gastroenterology, Hepatology and Nutrition June 21, 2012 OVERVIEW Definition Susceptibility The Changing Clinical Presentation Medical
Name of Policy: Human Leukocyte Antigen (HLA) Testing for Celiac Disease
 Name of Policy: Human Leukocyte Antigen (HLA) Testing for Celiac Disease Policy #: 545 Latest Review Date: June 2015 Category: Laboratory Policy Grade: B Background/Definitions: As a general rule, benefits
Name of Policy: Human Leukocyte Antigen (HLA) Testing for Celiac Disease Policy #: 545 Latest Review Date: June 2015 Category: Laboratory Policy Grade: B Background/Definitions: As a general rule, benefits
Eligibility The NCSF online quizzes are open to any currently certified fitness professional, 18 years or older.
 Eligibility The NCSF online quizzes are open to any currently certified fitness professional, 18 years or older. Deadlines Course completion deadlines correspond with the NCSF Certified Professionals certification
Eligibility The NCSF online quizzes are open to any currently certified fitness professional, 18 years or older. Deadlines Course completion deadlines correspond with the NCSF Certified Professionals certification
Alliance for Best Practice in Health Education
 Alliance for Best Practice in Health Education Objectives Following this program, participants will 1. List the clinical situations where celiac disease should be suspected 2. Distinguish between celiac
Alliance for Best Practice in Health Education Objectives Following this program, participants will 1. List the clinical situations where celiac disease should be suspected 2. Distinguish between celiac
A new approach to understand and control bitter pit in apple
 FINAL PROJECT REPORT WTFRC Project Number: AP-07-707 Project Title: PI: Organization: A new approach to understand and control bitter pit in apple Elizabeth Mitcham University of California Telephone/email:
FINAL PROJECT REPORT WTFRC Project Number: AP-07-707 Project Title: PI: Organization: A new approach to understand and control bitter pit in apple Elizabeth Mitcham University of California Telephone/email:
Vaccination for Celiac Disease: utopia or concrete hope for Celiac Disease recovery
 Vaccination for Celiac Disease: utopia or concrete hope for Celiac Disease recovery January 31, 2012 ImmusanT, Inc One Broadway, 14th Floor Cambridge, MA 02142 Key Points Objective: Treatment of celiac
Vaccination for Celiac Disease: utopia or concrete hope for Celiac Disease recovery January 31, 2012 ImmusanT, Inc One Broadway, 14th Floor Cambridge, MA 02142 Key Points Objective: Treatment of celiac
The immunopathogenesis of celiac disease reveals possible therapies beyond the gluten-free diet
 Semin Immunopathol (2012) 34:581 600 DOI 10.1007/s00281-012-0318-8 REVIEW ARTICLE The immunopathogenesis of celiac disease reveals possible therapies beyond the gluten-free diet Christopher S. McAllister
Semin Immunopathol (2012) 34:581 600 DOI 10.1007/s00281-012-0318-8 REVIEW ARTICLE The immunopathogenesis of celiac disease reveals possible therapies beyond the gluten-free diet Christopher S. McAllister
CELIAC DISEASE. Molly Jennings Deb McCafferty MS, RD
 CELIAC DISEASE Molly Jennings Deb McCafferty MS, RD WHAT IS CELIAC DISEASE? In short In this disease, exposure to gluten results in damge to the intestinal mucosa. Immune-mediated disorder Also known as
CELIAC DISEASE Molly Jennings Deb McCafferty MS, RD WHAT IS CELIAC DISEASE? In short In this disease, exposure to gluten results in damge to the intestinal mucosa. Immune-mediated disorder Also known as
Celiac Disease. Sheryl Pfeil, MD The Ohio State University Division of Gastroenterology, Hepatology, and Nutrition. January 2015
 Celiac Disease Sheryl Pfeil, MD The Ohio State University Division of Gastroenterology, Hepatology, and Nutrition January 2015 Objectives Review the clinical presentation of celiac disease, including intestinal
Celiac Disease Sheryl Pfeil, MD The Ohio State University Division of Gastroenterology, Hepatology, and Nutrition January 2015 Objectives Review the clinical presentation of celiac disease, including intestinal
Understanding Celiac Disease
 Understanding Celiac Disease Diagnostic Challenges Sheryl Pfeil, MD Professor of Clinical Medicine Division of Gastroenterology, Hepatology and Nutrition Department of Internal Medicine The Ohio State
Understanding Celiac Disease Diagnostic Challenges Sheryl Pfeil, MD Professor of Clinical Medicine Division of Gastroenterology, Hepatology and Nutrition Department of Internal Medicine The Ohio State
What is celiac disease?
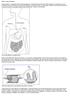 What is celiac disease? Celiac disease is a digestive disease that damages the small intestine and interferes with absorption of nutrients from food. People who have celiac disease cannot tolerate gluten,
What is celiac disease? Celiac disease is a digestive disease that damages the small intestine and interferes with absorption of nutrients from food. People who have celiac disease cannot tolerate gluten,
New Gluten World S.r.l. Carmen Lamacchia
 EURO GLOBAL SUMMIT AND EXPO ON FOOD AND BEVERAGES AN INNOVATIVE METHOD FOR THE DETOXIFICATION OF GLUTEN PROTEINS FROM GRAINS OF CEREALS New Gluten World S.r.l. Carmen Lamacchia Lead inventor and founder
EURO GLOBAL SUMMIT AND EXPO ON FOOD AND BEVERAGES AN INNOVATIVE METHOD FOR THE DETOXIFICATION OF GLUTEN PROTEINS FROM GRAINS OF CEREALS New Gluten World S.r.l. Carmen Lamacchia Lead inventor and founder
The immunology of gluten sensitivity: beyond the gut
 Opinion TRENDS in Immunology Vol.25 No.11 November 2004 The immunology of gluten sensitivity: beyond the gut Marios Hadjivassiliou 1, Clare A. Williamson 2 and Nicola Woodroofe 2 1 Department of Neurology,
Opinion TRENDS in Immunology Vol.25 No.11 November 2004 The immunology of gluten sensitivity: beyond the gut Marios Hadjivassiliou 1, Clare A. Williamson 2 and Nicola Woodroofe 2 1 Department of Neurology,
Understanding Food Intolerance and Food Allergy
 Understanding Food Intolerance and Food Allergy There are several different types of sensitivities or adverse reactions to foods. One type is known as a food intolerance ; an example is lactose intolerance.
Understanding Food Intolerance and Food Allergy There are several different types of sensitivities or adverse reactions to foods. One type is known as a food intolerance ; an example is lactose intolerance.
prevalence 181 Atopy patch test, see Patch test
 Subject Index AD, see Atopic dermatitis Adrenaline, anaphylaxis management 99 101, 194, 195 Adverse food reaction definition 4 nonallergic reactions 6, 9 Allergen Nomenclature database 20, 21 Allergen
Subject Index AD, see Atopic dermatitis Adrenaline, anaphylaxis management 99 101, 194, 195 Adverse food reaction definition 4 nonallergic reactions 6, 9 Allergen Nomenclature database 20, 21 Allergen
The Gluten Free Diet and Potential Alternative Therapies: The Road Ahead
 The Gluten Free Diet and Potential Alternative Therapies: The Road Ahead Daniel Leffler MD, MS Associate Professor of Medicine Harvard Medical School HARVARD MEDICAL SCHOOL Let Thy Food Be Thy Medicine
The Gluten Free Diet and Potential Alternative Therapies: The Road Ahead Daniel Leffler MD, MS Associate Professor of Medicine Harvard Medical School HARVARD MEDICAL SCHOOL Let Thy Food Be Thy Medicine
Understanding Celiac Disease
 Understanding Diagnostic Challenges Sheryl Pfeil, MD Professor of Clinical Medicine Division of Gastroenterology, Hepatology and Nutrition Department of Internal Medicine The Ohio State University Wexner
Understanding Diagnostic Challenges Sheryl Pfeil, MD Professor of Clinical Medicine Division of Gastroenterology, Hepatology and Nutrition Department of Internal Medicine The Ohio State University Wexner
L-Theanine Clinical Studies
 ALL A B C D E F G I K L M N O P Q R S T V Z L-Theanine Clinical Studies Nippon Nogei Kagakukaishi. Kobayashi K, et al. Effects of L-theanine on the release of a- brain waves in human volunteers. 1998;72(2):153-7.
ALL A B C D E F G I K L M N O P Q R S T V Z L-Theanine Clinical Studies Nippon Nogei Kagakukaishi. Kobayashi K, et al. Effects of L-theanine on the release of a- brain waves in human volunteers. 1998;72(2):153-7.
Wheat, Gluten and Health. WheatFoods.org
 Wheat, Gluten and Health WheatFoods.org Wheat: The Latest Dietary Villain Close to 30% of US adults* are interested in cutting down or avoiding gluten in their diets. And, most are not doing so out of
Wheat, Gluten and Health WheatFoods.org Wheat: The Latest Dietary Villain Close to 30% of US adults* are interested in cutting down or avoiding gluten in their diets. And, most are not doing so out of
Biomedical Sciences. 26 February Celiac Disease and Malabsorption. Prof. Dr. Christoph Mueller
 Biomedical Sciences 26 February 2014 Celiac Disease and Malabsorption Prof. Dr. Christoph Mueller Institute of Pathology christoph.mueller@pathology.unibe.ch Malabsorption Definition Malabsorption represents
Biomedical Sciences 26 February 2014 Celiac Disease and Malabsorption Prof. Dr. Christoph Mueller Institute of Pathology christoph.mueller@pathology.unibe.ch Malabsorption Definition Malabsorption represents
Coeliac disease catering gluten-free
 Coeliac disease catering gluten-free About Coeliac UK National Charity for people with coeliac disease and dermatitis herpetiformis Founded in 1968 and is the largest coeliac charity in the world Mission:
Coeliac disease catering gluten-free About Coeliac UK National Charity for people with coeliac disease and dermatitis herpetiformis Founded in 1968 and is the largest coeliac charity in the world Mission:
Saeeda Almarzooqi, 1 Ronald H. Houston, 2 and Vinay Prasad Introduction
 Pathology Research International Volume 2013, Article ID 602985, 5 pages http://dx.doi.org/10.1155/2013/602985 Clinical Study Utility of Tissue Transglutaminase Immunohistochemistry in Pediatric Duodenal
Pathology Research International Volume 2013, Article ID 602985, 5 pages http://dx.doi.org/10.1155/2013/602985 Clinical Study Utility of Tissue Transglutaminase Immunohistochemistry in Pediatric Duodenal
Licensing and gluten free markets in Estonia and other Nordic-Baltic countries. Katre Trofimov 2017
 Licensing and gluten free markets in Estonia and other Nordic-Baltic countries Katre Trofimov 2017 Who need gluten free food? Gluten-related disorders Coeliac disease blood markers + biopsy Dermatitis
Licensing and gluten free markets in Estonia and other Nordic-Baltic countries Katre Trofimov 2017 Who need gluten free food? Gluten-related disorders Coeliac disease blood markers + biopsy Dermatitis
FOOD ALLERGY AND MEDICAL CONDITION ACTION PLAN
 CAMPUS DINING AT HOLY CROSS COLLEGE FOOD ALLERGY AND MEDICAL CONDITION ACTION PLAN Accommodating Individualized Dietary Requirements Including Food Allergies, Celiac Disease, Intolerances, Sensitivities,
CAMPUS DINING AT HOLY CROSS COLLEGE FOOD ALLERGY AND MEDICAL CONDITION ACTION PLAN Accommodating Individualized Dietary Requirements Including Food Allergies, Celiac Disease, Intolerances, Sensitivities,
Celiac disease is a unique disorder that is both a food
 GASTROENTEROLOGY 2006;131:1981 2002 American Gastroenterological Association () Institute Technical Review on the Diagnosis and Management of Celiac Disease This technical review addresses the state of
GASTROENTEROLOGY 2006;131:1981 2002 American Gastroenterological Association () Institute Technical Review on the Diagnosis and Management of Celiac Disease This technical review addresses the state of
Where in the Genome is the Flax b1 Locus?
 Where in the Genome is the Flax b1 Locus? Kayla Lindenback 1 and Helen Booker 2 1,2 Plant Sciences Department, University of Saskatchewan, Saskatoon, SK S7N 5A8 2 Crop Development Center, University of
Where in the Genome is the Flax b1 Locus? Kayla Lindenback 1 and Helen Booker 2 1,2 Plant Sciences Department, University of Saskatchewan, Saskatoon, SK S7N 5A8 2 Crop Development Center, University of
Gluten and the skin: Celiac disease and gluten sensitivity for the dermatologist
 2/10/18 Gluten and the skin: Celiac disease and gluten sensitivity for the dermatologist 76th Annual American Academy of Dermatology Meeting February 16th, 2017 Matthew Goldberg, MD Assistant Professor,
2/10/18 Gluten and the skin: Celiac disease and gluten sensitivity for the dermatologist 76th Annual American Academy of Dermatology Meeting February 16th, 2017 Matthew Goldberg, MD Assistant Professor,
The miraculous power of Bulgarian yogurt. Created by LB BULGARICUM
 The miraculous power of Bulgarian yogurt HISTORY REMARKS Its secret is hidden in its micro-flora and the specific combination of strains from two species - Lactobacillus bulgaricus and Streptococcus thermophilus
The miraculous power of Bulgarian yogurt HISTORY REMARKS Its secret is hidden in its micro-flora and the specific combination of strains from two species - Lactobacillus bulgaricus and Streptococcus thermophilus
History of Food Allergies
 Grand Valley State University From the SelectedWorks of Jody L Vogelzang PhD, RDN, FAND, CHES Spring 2013 History of Food Allergies Jody L Vogelzang, PhD, RDN, FAND, CHES, Grand Valley State University
Grand Valley State University From the SelectedWorks of Jody L Vogelzang PhD, RDN, FAND, CHES Spring 2013 History of Food Allergies Jody L Vogelzang, PhD, RDN, FAND, CHES, Grand Valley State University
Celiac Disease. Definition & Facts. What is celiac disease? How common is celiac disease? Who is more likely to develop celiac disease?
 Celiac Disease Definition & Facts What is celiac disease? Celiac disease is a digestive disorder that damages the small intestine. The disease is triggered by eating foods containing gluten. Gluten is
Celiac Disease Definition & Facts What is celiac disease? Celiac disease is a digestive disorder that damages the small intestine. The disease is triggered by eating foods containing gluten. Gluten is
HOW LONG UNTIL TRULY GLUTEN-FREE?
 HOW LONG UNTIL TRULY GLUTEN-FREE? A TIMELINE FOR SELF-MANAGEMENT SKILL ACQUISITION IN ADULTS WITH CELIAC DISEASE Emma M. Clerx National Celiac Association Fall Meeting 10/29/2017 A LITTLE BIT ABOUT ME
HOW LONG UNTIL TRULY GLUTEN-FREE? A TIMELINE FOR SELF-MANAGEMENT SKILL ACQUISITION IN ADULTS WITH CELIAC DISEASE Emma M. Clerx National Celiac Association Fall Meeting 10/29/2017 A LITTLE BIT ABOUT ME
Celiac Disease. Gluten-Sensitive Enteropathy Celiac Sprue Non-tropical Sprue
 Celiac Disease Gluten-Sensitive Enteropathy Celiac Sprue Non-tropical Sprue Copyright 2017 by Sea Courses Inc. All rights reserved. No part of this document may be reproduced, copied, stored, or transmitted
Celiac Disease Gluten-Sensitive Enteropathy Celiac Sprue Non-tropical Sprue Copyright 2017 by Sea Courses Inc. All rights reserved. No part of this document may be reproduced, copied, stored, or transmitted
GUIDANCE ON THE DIAGNOSIS AND MANAGEMENT OF LACTOSE INTOLERANCE AND PRESCRIPTION OF LOW LACTOSE INFANT FORMULA.
 GUIDANCE ON THE DIAGNOSIS AND MANAGEMENT OF LACTOSE INTOLERANCE AND PRESCRIPTION OF LOW LACTOSE INFANT FORMULA. These are the lactose intolerance guidelines and it is recommended that they are used in
GUIDANCE ON THE DIAGNOSIS AND MANAGEMENT OF LACTOSE INTOLERANCE AND PRESCRIPTION OF LOW LACTOSE INFANT FORMULA. These are the lactose intolerance guidelines and it is recommended that they are used in
Esperanza Garcia-Alvarez MD Medical Director Pediatric Celiac Center at Advocate Children s Hospital
 Esperanza Garcia-Alvarez MD Medical Director Pediatric Celiac Center at Advocate Children s Hospital Nothing to disclose Objectives Better understanding pathogenesis celiac disease Better understanding
Esperanza Garcia-Alvarez MD Medical Director Pediatric Celiac Center at Advocate Children s Hospital Nothing to disclose Objectives Better understanding pathogenesis celiac disease Better understanding
WHY IS THERE CONTROVERSY ABOUT FOOD ALLERGY AND ECZEMA. Food Allergies and Eczema: Facts and Fallacies
 Food Allergies and Eczema: Facts and Fallacies Lawrence F. Eichenfield,, M.D. Professor of Clinical Pediatrics and Medicine (Dermatology) University of California, San Diego Rady Children s s Hospital,
Food Allergies and Eczema: Facts and Fallacies Lawrence F. Eichenfield,, M.D. Professor of Clinical Pediatrics and Medicine (Dermatology) University of California, San Diego Rady Children s s Hospital,
