February Monthly Update, Quest Diagnostics Nichols Institute, Valencia
|
|
|
- Fay Elliott
- 6 years ago
- Views:
Transcription
1 NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Code Test Name Effective Date Page # Cardio IQ Advanced Lipid Panel and Inflammation Panel 2/9/ Celiac Disease Comprehensive Panel 3/23/ Celiac Disease Comprehensive Panel, Infant 3/23/ Susceptibility, Aerobic Bacteria, MIC 3/23/ TEST CHANGES Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Code Former Test Code Test Name Effective Date Page # S46660 Antibody Screen, RBC with Reflex to Identification, Titer, and Antigen Typing 3/2/ S52113 HIV-1 Integrase Genotype 3/2/ , 4861I, 4861IX Lead, Blood (OSHA) 3/2/ Streptococcus Group A, DNA Probe 3/2/ Streptococcus Group B DNA, PCR with Broth Enrichment 3/2/ Streptococcus Group B DNA, PCR with Broth Enrichment and Reflex to Susceptibility 3/2/ Testosterone, Total, Males (Adult), Immunoassay 3/2/ S52037 Drug Screen Panel 9, Meconium 3/9/ Lynch Syndrome Sequencing and Deletion/Duplication Changes 3/9/ Lynch Syndrome, PMS2 Sequencing and Deletion/Duplication 3/9/ P5031F Custom Multicare Celiac Panel 3/23/ P48086G CVS Nav Transglutaminase & Celiac Panel 3/23/ Endomysial Antibody Screen (IgA) with Reflex to Titer 3/23/ S51352 Eosinophil Cationic Protein (ECP) 3/23/ Gliadin (Deamidated Peptide) Antibody (IgA) 3/23/ Gliadin (Deamidated Peptide) Antibody (IgG) 3/23/ Gliadin (Deamidated Peptide) Antibody (IgG, IgA) 3/23/ Reticulin IgA Screen with Reflex to Titer 3/23/ Tissue Transglutaminase Antibody (IgA) 3/23/ Tissue Transglutaminase Antibody (IgG) 3/23/ Tissue Transglutaminase Antibody (IgG,IgA) 3/23/ DISCONTINUED TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Page 1 of 24 The CPT Codes provided in this document are based on AMA guidelines and are for informational purposes only. CPT coding is the sole responsibility of the billing party. Please direct any questions regarding coding to the payor being billed. Any Profile/panel component may be ordered separately. Reflex tests are performed at an additional charge.
2 Test Code Test Name Effective Date Page # 1076 Celiac Disease AutoAbs Evaluation 3/23/ Celiac Disease EvaluatR w/iga 3/23/ Celiac Disease EvaluatR w/reflex to Titer 3/23/ P43321O Custom ADL Celiac Panel 3/23/ P8019B Custom ETCH Transglutaminase & Celiac Panel 3/23/ P6425A Custom QVMC Celiac Disease Panel 3/23/ P48580A Custom VA Roseburg Celiac Comprehensive Ab Panel 3/23/ SEND OUTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Code Former Test Code Test Name Effective Date Page # Hydroxyzine and Metabolite, S/P 3/2/ Endomysial IgG Antibody Screen and Titer 3/9/ New Test Offerings The following tests will be available through Quest Diagnostics on the dates indicated below. Cardio IQ Advanced Lipid Panel and Inflammation Panel Clinical Significance The 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults recommend matching the intensity of statin treatment with the absolute risk of cardiovascular events. However, the standard lipid panel alone does not provide a complete assessment of absolute risk of CVD. Adding advanced cvd markers (ion mobility, apob, lp(a), hs crp and lppla2) in addition to the lipid panel will improve assessment of cvd risk. Effective Date 2/9/2015 Test Code CPT Codes (82465, 83718, 84478), 83721, 83704, 82172, 83695, 86141, Specimen Requirements Instructions Transport Temperature Set-up/Analytic Time Reference Range 6 ml (3 ml minimum) serum Gross hemolysis; grossly lipemic; moderately and grossly icteric; samples stored and shipped room temperature See individual assays Refrigerated Room temperature and Frozen: Unacceptable Refrigerated: 5 days Set up: Mon-Fri; Report available: 3-6 days Cardio IQ Cholesterol, Total Pediatrics <20 years*: mg/dl <170 (Desirable) (Borderline) Page 2 of 24
3 > or = 200 (Higher Risk) Adults > or = 20 years**: mg/dl <200 (Desirable) (Borderline) > or = 240 (Higher Risk) References: * Pediatrics 1992 Mar, 89: ** An executive summary of the NCEP guidelines, the "Third Report of the NCEP Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults." Journal of the American Medical Association. May 16, 2001 Cardio IQ HDL Cholesterol Pediatric Reference Ranges for HDL Cholesterol**: Age Males Females <5 years: Reference range not established Reference range not established 5-14 years: mg/dl mg/dl years: mg/dl mg/dl Adult Reference Ranges for HDL Cholesterol***: > or = 20 years: > or = 40 mg/dl > or = 46 mg/dl References: ** Pediatrics 1992 Mar; 89: *** An executive summary of the NCEP guidelines, the "Third Report of the NCEP Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults." Journal of the American Medical Association. May 16, 2001 Cardio IQ Triglycerides Pediatric Reference Ranges for Triglycerides*: Age Males Females Birth-9 years: mg/dl mg/dl years: mg/dl mg/dl years: mg/dl mg/dl Adult Reference Ranges for Triglycerides**: <150 mg/dl (Normal) mg/dl (Borderline-High) mg/dl (High) > or = 500 mg/dl (Very High) References: * Pediatrics 1992 Mar, 89: ** An executive summary of the NCEP guidelines, the "Third Report of the NCEP Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults." Journal of the American Medical Association. May 16, 2001 Cardio IQ Non-HDL and Calculated Components LDL Cholesterol, Calculated Page 3 of 24
4 Pediatric Reference Ranges for LDL Cholesterol (2-20 years): <110 (Desirable) (Borderline) > or = 130 (High) Adult Reference Ranges for LDL Cholesterol: <130 (Desirable) (Borderline) > or = 160 (High) Desirable Range <100 mg/dl for patients with CHD or Diabetes and <70 mg/dl for Diabetic patients with known heart disease. Cholesterol/HDL Ratio: < or = 5.0 calc Non-HDL Cholesterol: <20 years: <120 mg/dl > or = 20 years: Target for non-hdl cholesterol is 30 mg/dl higher than LDL cholesterol target. Cardio IQ Direct LDL Reference Ranges for Direct LDL: <20 years: <110 mg/dl > or = 20 years: <130 mg/dl Desirable Range <100 mg/dl for patients with CHD or Diabetes and <70 mg/dl for Diabetic patients with known heart disease. Cardio IQ Lipoprotein Fractionation, Ion Mobility LDL Particle Number: Males and Females: nmol/l Risk: Optimal <1260; Moderate ; High >1538 LDL Small: Males: Females: nmol/l nmol/l Risk: Optimal <162; Moderate ; High >217 LDL Medium: Males: Females: nmol/l nmol/l Risk: Optimal <201; Moderate ; High >271 HDL Large: Males: Females: nmol/l nmol/l Risk: Optimal >9386; Moderate ; High <6996 Page 4 of 24
5 LDL Pattern: A Pattern Risk: Optimal Pattern A; High Pattern B LDL Peak Size: > or = Angstrom Risk: Optimal >222.5; Moderate ; High <218.2 Adult cardiovascular event risk category cut points (optimal, moderate, high) are based on adult U.S. reference population. Association between lipoprotein subfractions and cardiovascular events is based on Musunuru et al. ATVB. 2009;29:1975 Cardio IQ Apolipoprotein B Adult Males: Adult Females: mg/dl mg/dl Risk: Optimal < 80 mg/dl; Moderate mg/dl; High > or = 120 mg/dl Cardiovascular event risk category cut points (optimal, moderate, high) are based on National Lipid Association recommendations - Davidison et al. J Clin Lipidol. 2011;5:338 Cardio IQ Lipoprotein (a) Lipoprotein (a): <75 nmol/l Risk: Optimal < 75 nmol/l; Moderate nmol/l; High > 125 nmol/l Cardiovascular event risk category cut points (optimal, moderate, high) are based on Marcovina et al. Clin Chem. 2003;49:1785 and Nordestgaard et al. European Heart J. 2010;31:2844 (results of metaanalysis and expert panel recommendations) Cardio IQ hs-crp hs-crp for ages >17 years: Risk According to AHA/CDC Guidelines <1.0 mg/l Lower Relative Cardiovascular Risk mg/l Average Relative Cardiovascular Risk mg/l Higher Relative Cardiovascular Risk. Consider retesting in 1 to 2 weeks to exclude a benign transient elevation in the baseline CRP value secondary to infection or inflammation. >10.0 mg/l Persistent elevations upon retesting, may be associated with infection and inflammation. Cardio IQ Lp-PLA2 (PLAC ) PLAC: ng/ml Risk: Optimal < 200 ng/ml; Moderate ng/ml; High > 235 ng/ml Cardiovascular event risk category cut points (optimal, moderate, high) are based on Lanman et al. Prev Cardiol. 2006;9:138 Methodology Spectrophotometry; Enzymatic; Ion Mobility; Nephelometry; Immunoturbidimetric; Immunoassay Quest Diagnostics Nichols Institute, San Juan Capistrano Cholesterol, Total HDL Cholesterol Triglycerides LDL Chol, Calculated Page 5 of 24
6 Cholesterol/HDL Ratio Non-HDL Cholesterol LDL Particle Number LDL Small LDL Medium HDL Large LDL Pattern LDL Peak Size Apolipoprotein B Lipoprotein (a) hs-crp PLAC *TR Cardio IQ(R) Direct LDL Direct LDL *TR (True Reflexing Flag) Interfaced clients: If you are set up to use our True Reflexing option, build the unit code with the TR flag (indicated above) separately. Additional Information If Triglyceride is >400 mg/dl, then Cardio IQ Direct LDL will be performed at an additional charge (CPT code(s): 83721). Celiac Disease Comprehensive Panel Clinical Significance Celiac disease is caused by an immune response to gluten in genetically sensitive individuals. The diagnosis is largely based on a biopsy of the small intestine, but serologic tests also help support a diagnosis and may assist identification of patients who may require biopsy. Tissue transglutaminase antibodies (ttg, IgA) is a marker with 95% sensitivity and specificity. Total IgA is measured because 2-3% of celiac disease patients are IgA deficient. Because ttg, IgA, and anti-gliadin IgA tend to decrease in patients on a gluten-free diet, these markers are also used to assess dietary compliance. The endomysial antibody (EMA, IgA) assay has high specificity for celiac disease and is used to confirm positive anti-ttg results. Test Code CPT Codes 83516, Specimen Requirements Transport Temperature Set-up/Analytic Time 5 ml (1 ml minimum) serum Gross hemolysis; gross lipemia Refrigerated Room temperature: 72 hours Refrigerated: 7 days Frozen: 21 days Set up: Daily; report available: 1-6 days Page 6 of 24
7 Reference Range (ttg) Ab, IgA: < 4 No Antibody Detected > or = 4 Antibody Detected IgA, Serum: Cord Blood: Month: Months: Months: Months: Years: Years: Years: Years: Years: Years: >=16 Years: Unit of Measure Interpretation (ttg) Ab, IgA U/mL IgA, Serum mg/dl This is a true reflex. Please build the unit code separately. Non-orderable Reflex: RUF- Reflex Endomysial Ab IgA Screen Endomysial Ab IgA This is a true reflex. Please build the unit code separately. Non-orderable Reflex: RUG- Reflex Endomysial Ab IgA Titer Endomysial Ab Titer This is a true reflex. Please build the unit code separately. Non-orderable Reflex: RUH- Reflex Tissue Transglutaminase IgG (ttg) Ab, IgG Additional Information If the Tissue Transglutaminase IgA is positive, then Endomysial Antibody Screen (IgA) will be performed at an additional charge. If the Endomysial Antibody Screen (IgA) is positive, then Endomysial Antibody Titer will be performed at an additional charge. If the Total IgA is less than the lower limit of the reference range, based on age, then Tissue Transglutaminase IgG will be performed at an additional charge. Celiac Disease Comprehensive Panel, Infant Clinical Significance Celiac disease is caused by an immune response to gluten in genetically sensitive individuals. The diagnosis is largely based on a biopsy of the small intestine, but serologic tests also help support a diagnosis and may assist identification of patients who may require biopsy. Tissue transglutaminase antibodies (ttg, IgA) is a marker with 95% sensitivity and specificity. Total IgA is measured because 2-3% of celiac disease patients are IgA deficient. Because ttg, IgA, and anti-gliadin IgA tend to decrease in patients on a gluten-free diet, these markers are also used to assess dietary compliance. The endomysial antibody (EMA, IgA) assay has high specificity for celiac disease and is used to confirm positive anti-ttg results. Page 7 of 24
8 Test Code CPT Codes (x2), Specimen Requirements Transport Temperature Set-up/Analytic Time Reference Range 5 ml (1 ml minimum) serum Gross hemolysis; gross lipemia Refrigerated Room temperature: 72 hours Refrigerated: 7 days Frozen: 21 days Set up: Daily; report available: 1-6 days (ttg) Ab, IgA: < 4 No Antibody Detected > or = 4 Antibody Detected IgA, Serum: Cord Blood: Month: Months: Months: Months: Years: Years: Years: Years: Years: Years: >=16 Years: Unit of Measure Interpretation (ttg) Ab, IgA U/mL IgA, Serum mg/dl This is a true reflex. Please build the unit code separately. Non-orderable Reflex: RUF- Reflex Endomysial Ab IgA Screen Endomysial Ab IgA This is a true reflex. Please build the unit code separately. Non-orderable Reflex: RUG- Reflex Endomysial Ab IgA Titer Endomysial Ab Titer This is a true reflex. Please build the unit code separately. Non-orderable Reflex: RUH- Reflex Tissue Transglutaminase IgG (ttg) Ab, IgG Additional Information If the Tissue Transglutaminase IgA is positive, then Endomysial Antibody Screen (IgA) will be performed at an additional charge. If the Endomysial Antibody Screen (IgA) is positive, then Endomysial Antibody Page 8 of 24
9 Titer will be performed at an additional charge. If the Total IgA is less than the lower limit of the reference range, based on age, then Tissue Transglutaminase IgG will be performed at an additional charge. Susceptibility, Aerobic Bacteria, MIC Test Code CPT Codes Specimen Requirements Transport Temperature Set-up/Analytic Time Reference Range Units Of Measure Methodology Pure culture of an aerobic organism submitted on an agar slant, double-walled container Mixed culture; anaerobic organism Room temperature Room temperature and Refrigerated: Determined by viability Frozen: Unacceptable Set up: Daily; Report available: 5 days Accompanies report mcg/ml Microbroth Dilution Type Prompt-Result Specimen Source: Prompt-Result Organism Amikacin Amoxicillin/clavulanic ACD Ampicillin/sulbactam Ampicillin Aztreonam Cefazolin Cefepime Cefoxitin Ceftaroline Ceftazidime Ceftriaxone Cefuroxime Chloramphenicol Ciprofloxacin Clarithromycin Clindamycin Page 9 of 24
10 Daptomycin Doripenem Ertapenem Erythromycin Gentamicin Gentamicin Imipenem Levofloxacin Linezolid Meropenem Moxifloxacin Nitrofurantoin Oxacillin Penicillin Piperacillin/tazobactam Trimethoprim/sulfamethoxaz Tetracycline Ticarcillin/clavulanic ACD Tigecycline Tobramycin Vancomycin Comment: Test Changes The following test changes will be effective on the dates indicated below. Please note information that is changing appears in bold text in this update. Former test names and test codes have been italicized. Antibody Screen, RBC with Reflex to Identification, Titer, and Antigen Typing Effective Date 3/2/2015 Former Test Name Test Code Always Message Antibody Screen RBC S46660 Gross hemolysis; grossly lipemic; received frozen; serum separator tube (SST); cord blood; grossly icteric Room temperature: 24 hours Refrigerated: 7 days Frozen: Unacceptable This assay is a screening test for the detection of red blood cell antibodies. The test is not to be used for pretransfusion screening or for the medical management of an alloimmunized pregnancy. Page 10 of 24
11 Quest Diagnostics, West Hills HIV-1 Integrase Genotype Effective Date 3/2/2015 Former Test Code S52113 Test Code Set-up/Analytic Time Serum; non-centrifuged PPT; frozen PPT (in situ); heparinized plasma; gross hemolysis; lipemia Set up: Mon, Fri; Report available: 4-7 days This test previously performed at Quest Diagnostics Nichols Institute, San Juan Capistrano will now be performed at Focus Diagnostics, Inc. Type Unit of Measure Prompt-Result Value of Last Viral Load copies/ml Prompt-Result Date Viral Load Collected Raltegravir Resistance Elvitegravir Resistance Dolutegravir Resistance Lead, Blood (OSHA) Effective Date 3/2/2015 Test Code 3058, 4861I, 4861IX Type Lead, Blood (OSHA) Prompt-Result Date of Birth Prompt-Result (no return) Gazetteer Code Prompt-Result (no return) Patient Race Prompt-Result (no return) Ethnicity Prompt-Result (no return) Venous/Capillary Prompt-Result (no return) Patient Street Address Prompt-Result (no return) Patient City Prompt-Result (no return) Patient State Prompt-Result (no return) Patient Zip Code Prompt-Result (no return) Patient County Prompt-Result (no return) Patient Phone Number Prompt-Result (no return) Patient Occupation Page 11 of 24
12 Prompt-Result (no return) Employment Status Prompt-Result (no return) Employer Prompt-Result (no return) Employer Address Prompt-Result (no return) Employer City Prompt-Result (no return) Employer State Prompt-Result (no return) Employer Zip Prompt-Result (no return) Employer Phone Prompt-Result (no return) Purpose of Test Prompt-Result (no return) Parent's Last Name Prompt-Result (no return) Parent's First Name Prompt-Result (no return) Parent's Phone Number Prompt-Result (no return) Medical Provider Prompt-Result (no return) Provider's Street Address Prompt-Result (no return) Provider's City Prompt-Result (no return) Provider's State Prompt-Result (no return) Provider's Zip Code Prompt-Result (no return) Provider's Phone Number Additional Information Update report format Streptococcus Group A, DNA Probe Effective Date 3/2/2015 Test Code Deliver to lab as soon as possible Room temperature: 48 hours Refrigerated: 72 hours Frozen: Unacceptable Focus Diagnostics, Inc. Streptococcus Group B DNA, PCR with Broth Enrichment Effective Date 3/2/2015 Test Code Assay Category FDA Approved/Cleared This test previously performed at Focus Diagnostics, Inc. will now also be performed at Quest Diagnostics Nichols Institute, Valencia. Streptococcus Group B DNA, PCR with Broth Enrichment and Reflex to Susceptibility Effective Date 3/2/2015 Page 12 of 24
13 Test Code Assay Category FDA Approved/Cleared This test previously performed at Focus Diagnostics, Inc. will now also be performed at Quest Diagnostics Nichols Institute, Valencia. Type Prompt-Result Specimen Source: Group B Streptococcus This is a true reflex. Please build the unit code below aeparately. Non-orderable Reflex: RUK- Reflex Suscep., Aerobic Bacteria, MIC Type Prompt-Result Specimen Source: Prompt-Result Organism Amikacin Amoxicillin/clavulanic ACD Ampicillin/sulbactam Ampicillin Aztreonam Cefazolin Cefepime Cefoxitin Ceftaroline Ceftazidime Ceftriaxone Cefuroxime Chloramphenicol Ciprofloxacin Clarithromycin Clindamycin Daptomycin Doripenem Ertapenem Erythromycin Gentamicin Gentamicin Imipenem Levofloxacin Linezolid Page 13 of 24
14 Meropenem Moxifloxacin Nitrofurantoin Oxacillin Penicillin Piperacillin/tazobactam Trimethoprim/sulfamethoxaz Tetracycline Ticarcillin/clavulanic ACD Tigecycline Tobramycin Vancomycin Comment: Additional Information If GBS by PCR is Detected, Susceptibility, Aerobic Bacteria, MIC will be added at an additional charge (CPT code: 87186). Testosterone, Total, Males (Adult), Immunoassay Effective Date 3/2/2015 Former Test Name Testosterone, Total(Males), Immunoassay Test Code Reference Range Male: ng/dl Female: Reference range not applicable ng/dl Testosterone,Tot,MaleAdult Drug Screen Panel 9, Meconium Effective Date 3/9/2015 Test Code CPT Codes S (x9) Quest Diagnostics Nichols Institute, Chantilly Unit of Measure Opiates Page 14 of 24
15 Cocaine Metabolites Benzodiazepines Marijuana Amphetamines Barbiturates Methadone PCP (Phencyclidine) Propoxyphene A Codeine ng/g B Morphine ng/g E Hydrocodone ng/g D Hydromorphone ng/g F Oxycodone ng/g A Cocaine ng/g BB Benzoylecgonine ng/g BC Cocaethylene ng/g BD Ecgonine methyl ester ng/g Oxazepam ng/g Nordiazepam ng/g Desalkylflurazepam ng/g Lorazepam ng/g Alprazolam ng/g B Delta-9-THC Carboxy Acid ng/g A Amphetamine ng/g B Methamphetamine ng/g Butalbital mcg/g Butabarbital mcg/g Amobarbital mcg/g Pentobarbital mcg/g Secobarbital mcg/g Phenobarbital ng/g Methadone ng/g 91464A Phencyclidine ng/g Propoxyphene ng/g Norpropoxyphene ng/g Comment Page 15 of 24
16 Lynch Syndrome Sequencing and Deletion/Duplication Changes Effective Date 3/9/2015 Specimen Requirements Preferred: 4 ml (4 ml minimum) whole blood collected in each of 2 separate EDTA (lavender-top) tubes Acceptable: whole blood collected in each of 2 separate ACD tubes Quest Diagnostics Nichols Institute, San Juan Capistrano Tests Affected Test Codes: Name: Lynch Syndrome Panel Lynch Syndrome, MLH1 Sequencing and Deletion/Duplication Lynch Syndrome, MSH2 Sequencing and Deletion/Duplication (Including EPCAM) Lynch Syndrome, MSH6 Sequencing and Deletion/Duplication Lynch Syndrome, PMS2 Sequencing and Deletion/Duplication Lynch Syndrome, PMS2 Sequencing and Deletion/Duplication Effective Date 3/9/2015 Test Code Specimen Requirements Preferred: 4 ml (4 ml minimum) whole blood collected in each of two separate EDTA (lavender-top) tubes Acceptable: whole blood collected in each of two separate ACD tubes Sodium heparin (green-top) tubes are no longer acceptable Quest Diagnostics Nichols Institute, San Juan Capistrano Custom Multicare Celiac Panel Test Code P5031F Gross hemolysis; gross lipemia Room temperature: 4 Days Refrigerated and Frozen: 21 Days Endomysial Ab IgA Reticulin IgA Screen This is a true reflex. Please build code separately. Non-orderable Reflex: RUG-Reflex Endomysial Ab Titer Page 16 of 24
17 Endomysial Ab Titer This is a true reflex. Please build code separately. Non-orderable Reflex: RUI- Reflex Reticulin IgA Titer Reticulin IgA Titer Additional Information If Endomysial Antibody IgA Screen is positive, the antibody titer will be added on at an additional charge (CPT: 86256). If Reticulin IgA Screen is positive, the antibody titer will be added on at an additional charge (CPT: 86256). CVS Nav Transglutaminase & Celiac Panel Test Code Reference Range P48086G Microbially contaminated serum; gross hemolysis; gross lipemia Room temperature: 48 hours Refrigerated: 7 days Frozen: 21 days (ttg) Ab, IgG: < 6 No Antibody Detected > or = 6 Antibody Detected (ttg) Ab, IgA: < 4 No Antibody Detected > or = 4 Antibody Detected Reticulin IgA Screen: Negative Gliadin(Deamidated)Ab,IgG: < 20 Gliadin(Deamidated)Ab,IgA: < 20 Endomysial Ab IgA: Negative Unit of Measure (ttg) Ab, IgG U/mL (ttg) Ab, IgA U/mL Gliadin(Deamidated)Ab,IgG U Gliadin(Deamidated)Ab,IgA U Reticulin IgA Screen Endomysial Ab IgA This is a true reflex. Please build code separately. Non-orderable Reflex: RUI- Reflex Reticulin IgA Titer Reticulin IgA Titer This is a true reflex. Please build code separately. Non-orderable Reflex: RUG- Reflex Endomysial Ab Titer Page 17 of 24
18 Endomysial Ab Titer Additional Information If Endomysial Antibody IgA Screen is positive, the antibody titer will be added on at an additional charge (CPT: 86256). If Reticulin IgA Screen is positive, the antibody titer will be added on at an additional charge (CPT: 86256). Endomysial Antibody Screen (IgA) with Reflex to Titer Former Test Code 1191 Test Code Gross hemolysis; gross lipemia Room temperature: 4 Days Refrigerated and Frozen: 21 Days Endomysial Ab IgA This is a true reflex. Please build code separately. Non-orderable Reflex: RUG-Reflex Endomysial Ab Titer Endomysial Ab Titer Additional Information If Endomysial Antibody IgA Screen is positive, the antibody titer will be added on at an additional charge (CPT: 86256) Eosinophil Cationic Protein (ECP) Test Code Specimen Requirements Set-up/Analytic Time S ml (0.3 ml minimum) serum collected in a red-top (no gel) tube Serum Separator Tube Set up: Tues; Report available: 1-4 days Quest Diagnostics Nichols Institute, San Juan Capistrano Gliadin (Deamidated Peptide) Antibody (IgA) Clinical Significance Detection of antibodies to gliadin, one of the major protein components of gluten, is a sensitive assay useful in diagnosing Celiac Disease. However, gliadin antibodies may be found in individuals without Celiac Disease; thus gliadin antibody assays are less specific than assays measuring antibodies to endomysium and transglutaminase. Recent work has revealed that gliadin-reactive antibodies from Celiac patients bind to a very limited number of specific epitopes on the gliadin molecule. Further, deamidation of gliadin results in enhanced binding of gliadin antibodies. Based on this information, assays using deamidated gliadin peptides bearing the celiac-specific epitopes have much higher diagnostic accuracy for Celiac Disease when compared to standard gliadin antibody assays. Page 18 of 24
19 Former Test Code 1286 Test Code Microbially contaminated serum; gross hemolysis; gross lipemia. Unit of Measure Gliadin(Deamidated)Ab,IgA U Gliadin (Deamidated Peptide) Antibody (IgG) Clinical Significance Detection of antibodies to gliadin, one of the major protein components of gluten, is a sensitive assay useful in diagnosing Celiac Disease. However, gliadin antibodies may be found in individuals without Celiac Disease; thus gliadin antibody assays are less specific than assays measuring antibodies to endomysium and transglutaminase. Recent work has revealed that gliadin-reactive antibodies from Celiac patients bind to a very limited number of specific epitopes on the gliadin molecule. Further, deamidation of gliadin results in enhanced binding of gliadin antibodies. Based on this information, assays using deamidated gliadin peptides bearing the celiac-specific epitopes have much higher diagnostic accuracy for Celiac Disease when compared to standard gliadin antibody assays. Former Test Code 1261 Test Code Microbially contaminated serum; gross hemolysis; gross lipemia Unit of Measure Gliadin(Deamidated)Ab,IgG U Gliadin (Deamidated Peptide) Antibody (IgG, IgA) Clinical Significance Detection of antibodies to gliadin, one of the major protein components of gluten, is a sensitive assay useful in diagnosing Celiac Disease. However, gliadin antibodies may be found in individuals without Celiac Disease; thus gliadin antibody assays are less specific than assays measuring antibodies to endomysium and transglutaminase. Recent work has revealed that gliadin-reactive antibodies from Celiac patients bind to a very limited number of specific epitopes on the gliadin molecule. Further, deamidation of gliadin results in enhanced binding of gliadin antibodies. Based on this information, assays using deaminated gliadin peptides bearing the celiac-specific epitopes have much higher diagnostic accuracy for Celiac Disease when compared to standard gliadin antibody assays. Former Test Code 1266 Test Code 8889 Microbially contaminated serum; gross hemolysis; gross lipemia Unit of Measure Gliadin(Deamidated)Ab,IgG U Page 19 of 24
20 Gliadin(Deamidated)Ab,IgA U Reticulin IgA Screen with Reflex to Titer Former Test Code 1162 Test Code Methodology Gross hemolysis; hyperlipemia; post mortem specimens Room temperature: 7 days Refrigerated: 14 days Frozen: 30 days Immunoassay Reticulin IgA Screen This is a true reflex. Please build code separately. Non-orderable Reflex: RUI- Reflex Reticulin IgA Titer Reticulin IgA Titer Additional Information If Reticulin IgA Screen is positive, the antibody titer will be added on at an additional charge (CPT: 86256) Tissue Transglutaminase Antibody (IgA) Clinical Significance Celiac Disease is characterized by gluten intolerance lading to a chronic malabsorptive disorder due to inflammation of the intestinal mucosa and flattening of the epithelium. Several studies demonstrated that the target endomysial antigen in IgA anti-gliadin and anti-reticulin assays has been identified as the calcium dependent, protein cross-linking, enzyme tissue transglutaminase. Former Test Name Transglutaminase IgA Autoantibodies Former Test Code 1029 Test Code 8821 Gross hemolysis; gross lipemia Room temperature: 4 days Refrigerated: 7 days Frozen: 30 days Reference Range < 4 No Antibody Detected > or = 4 Antibody Detected Unit of Measure (ttg) Ab, IgA U/mL Page 20 of 24
21 Tissue Transglutaminase Antibody (IgG) Clinical Significance Celiac Disease is characterized by gluten intolerance leading to a chronic malabsorptive disorder due to inflammation of the intestinal mucosa and flattening of the epithelium. Several studies have demonstrated that the target endomysial antigen in IgA anti-gliadin and anti-reticulin assays has been identified as the calcium dependent, protein cross-linking, enzyme tissue transglutaminase. Former Test Name Transglutaminase IgG Autoantibodies Former Test Code 1027 Test Code Room temperature: 4 days Refrigerated: 7 days Frozen: 30 days Reference Range < 6 No Antibody Detected > or = 6 Antibody Detected Unit of Measure (ttg) Ab, IgG U/mL Tissue Transglutaminase Antibody (IgG,IgA) Clinical Significance Celiac Disease is characterized by gluten intolerance leading to a chronic malabsorptive disorder due to inflammation of the intestinal mucosa and flattening of the epithelium. Several studies have demonstrated that the target endomysial antigen in IgA anti-gliadin and anti-reticulin assays has been identified as the calcium dependent, protein cross-linking, enzyme tissue transglutaminase. Former Test Name Transglutaminase IgG & IgA Autoantibodies Former Test Code 1030 Test Code Specimen Requirements Reference Range 1 ml (0.5 ml minimum) serum Gross hemolysis; gross lipemia Room temperature: 4 days Refrigerated: 7 days Frozen: 30 days (ttg) Ab, IgG: < 6 No Antibody Detected > or = 6 Antibody Detected (ttg) Ab, IgA: < 4 No Antibody Detected > or = 4 Antibody Detected Unit of Measure (ttg) Ab, IgG U/mL Page 21 of 24
22 (ttg) Ab, IgA U/mL Discontinued Tests Celiac Disease AutoAbs Evaluation Test Code 1076 Additional Information The recommended alternatives are dependant on the patient's age, test codes: Celiac Disease Comprehensive Panel -or Celiac Disease Comprehensive Panel, Infant Celiac Disease EvaluatR w/iga Test Code 1075 Additional Information The recommended alternatives are dependant on the patient's age, test codes: Celiac Disease Comprehensive Panel -or Celiac Disease Comprehensive Panel, Infant Celiac Disease EvaluatR w/reflex to Titer Test Code 1077 Additional Information The recommended alternatives are dependant on the patient's age, test codes: Celiac Disease Comprehensive Panel -or Celiac Disease Comprehensive Panel, Infant Custom ADL Celiac Panel Test Code Additional Information P43321O Due to low volume this test is being discontinued. There is no recommended alternative. Custom ETCH Transglutaminase & Celiac Panel Test Code Additional Information P8019B Due to low volume this test is being discontinued. There is no recommended alternative. Page 22 of 24
23 Custom QVMC Celiac Disease Panel Test Code Additional Information P6425A Due to low volume this test is being discontinued. There is no recommended alternative. Custom VA Roseburg Celiac Comprehensive Ab Panel Test Code Additional Information P48580A Due to low volume this test is being discontinued. There is no recommended alternative. Test Send Outs (Referrals) Hydroxyzine and Metabolite, S/P Effective Date 3/2/2015 Test Code Set-up/Analytic Time Room temperature and Refrigerated: 30 days Frozen: 2 years Set up: Mon, Wed, Fri 2 nd shift; Report available: 3 days Endomysial IgG Antibody Screen and Titer Message Clinical Significance **This test is not available for New York patient testing** Serological methods of detecting Immunoglobulin A (IgA) antibodies to gliadin, endomysium (EMA), reticulin, and tissue transglutaminase are routinely used for diagnosing both symptomatic and asymptomatic patients with Celiac Disease (CD). Since Immunoglobulin A (IgA) deficiency is 10 to 15 times more common in patients with Celiac Disease than in healthy subjects, IgG-specific antibody tests for endomysium are useful for the identification of IgA-deficient patients with CD. Effective Date 3/9/2015 Test Code CPT Codes Specimen Requirements Preferred: 2 ml (0.2 ml minimum) serum collected in a red-top tube (no-gel) Acceptable: Serum separator tube Instructions Transport Temperature Gross hemolysis, lipemia, microbially contaminated specimens; specimens received outside of stability Allow the blood to clot in an upright position for at least 30 minutes but not longer than 1 hour before centrifugation. Centrifuge for at least 15 minutes at RPM at room temperature within one hour of collection, store at -20 C, and send 2 ml of serum frozen in a plastic vial. Frozen Room temperature: 48 hours Refrigerated: 14 days Frozen: 30 days Page 23 of 24
24 Set-up/Analytic Time Reference Range Methodology Set up: Mon-Fri; Report available: 1-9 Days Negative Immunofluorescence Assay Endomysial IgG Titer Additional Information Endomysial IgG Titer will report when the titer is > or = 2.5 (CPT code(s): 86256) Page 24 of 24 Quest, Quest Diagnostics, the associated logo, Nichols Institute and all associated Quest Diagnostics marks are the trademarks of Quest Diagnostics Quest Diagnostics Incorporated. All rights reserved.
March Monthly Update, Quest Diagnostics Nichols Institute, Valencia
 TEST CHANGES Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Code Former Test Code Test
TEST CHANGES Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Code Former Test Code Test
DEAMIDATED GLIADIN PEPTIDES IN COELIAC DISEASE DIAGNOSTICS
 DEAMIDATED GLIADIN PEPTIDES IN COELIAC DISEASE DIAGNOSTICS Z. Vanickova 1, P. Kocna 1, K. Topinkova 1, M. Dvorak 2 1 Institute of Clinical Biochemistry & Laboratory Diagnostics; 2 4th Medical Department,
DEAMIDATED GLIADIN PEPTIDES IN COELIAC DISEASE DIAGNOSTICS Z. Vanickova 1, P. Kocna 1, K. Topinkova 1, M. Dvorak 2 1 Institute of Clinical Biochemistry & Laboratory Diagnostics; 2 4th Medical Department,
See Policy CPT CODE section below for any prior authorization requirements
 Effective Date: 1/1/2019 Section: LAB Policy No: 404 Medical Policy Committee Approved Date: 12/17; 12/18 1/1/19 Medical Officer Date APPLIES TO: All lines of business See Policy CPT CODE section below
Effective Date: 1/1/2019 Section: LAB Policy No: 404 Medical Policy Committee Approved Date: 12/17; 12/18 1/1/19 Medical Officer Date APPLIES TO: All lines of business See Policy CPT CODE section below
The first and only fully-automated, random access, multiplex solution for Celiac IgA and Celiac IgG autoantibody testing.
 Bio-Rad Laboratories bioplex 2200 SYSTEM BioPlex 2200 Celiac IgA and IgG Kits * The first and only fully-automated, random access, multiplex solution for Celiac IgA and Celiac IgG autoantibody testing.
Bio-Rad Laboratories bioplex 2200 SYSTEM BioPlex 2200 Celiac IgA and IgG Kits * The first and only fully-automated, random access, multiplex solution for Celiac IgA and Celiac IgG autoantibody testing.
The first and only fully-automated, random access, multiplex solution for Celiac IgA and Celiac IgG autoantibody testing.
 Bio-Rad Laboratories BIOPLEX 2200 SYSTEM BioPlex 2200 Celiac IgA and IgG Kits The first and only fully-automated, random access, multiplex solution for Celiac IgA and Celiac IgG autoantibody testing. The
Bio-Rad Laboratories BIOPLEX 2200 SYSTEM BioPlex 2200 Celiac IgA and IgG Kits The first and only fully-automated, random access, multiplex solution for Celiac IgA and Celiac IgG autoantibody testing. The
SPECIMEN REQUIREMENTS. Specimen Specimen Volume (min) Specimen Type Specimen Container Transport Environment
 Carboxyhemoglobin Order Name: CARBOXYHGB Test Number: 2001600 Revision Date: 09/24/2014 LOINC Code: 20563-3 Carboxyhemoglobin Hemoximeter Preferred 2 ml (1.0) Whole Blood Lithium Heparin (Dark Green Top
Carboxyhemoglobin Order Name: CARBOXYHGB Test Number: 2001600 Revision Date: 09/24/2014 LOINC Code: 20563-3 Carboxyhemoglobin Hemoximeter Preferred 2 ml (1.0) Whole Blood Lithium Heparin (Dark Green Top
Diagnostic Testing Algorithms for Celiac Disease
 Diagnostic Testing Algorithms for Celiac Disease HOT TOPIC / 2018 Presenter: Melissa R. Snyder, Ph.D. Co-Director, Antibody Immunology Laboratory Department of Laboratory Medicine and Pathology, Mayo Clinic
Diagnostic Testing Algorithms for Celiac Disease HOT TOPIC / 2018 Presenter: Melissa R. Snyder, Ph.D. Co-Director, Antibody Immunology Laboratory Department of Laboratory Medicine and Pathology, Mayo Clinic
Diagnosis Diagnostic principles Confirm diagnosis before treating
 Diagnosis 1 1 Diagnosis Diagnostic principles Confirm diagnosis before treating Diagnosis of Celiac Disease mandates a strict gluten-free diet for life following the diet is not easy QOL implications Failure
Diagnosis 1 1 Diagnosis Diagnostic principles Confirm diagnosis before treating Diagnosis of Celiac Disease mandates a strict gluten-free diet for life following the diet is not easy QOL implications Failure
November Laboratory Testing for Celiac Disease. Inflammation in Celiac Disease
 November 2011 Gary Copland, MD Chair, Department of Pathology, Unity Hospital Laboratory Medical Director, AMC Crossroads Chaska and AMC Crossroads Dean Lakes Laboratory Testing for Celiac Disease Celiac
November 2011 Gary Copland, MD Chair, Department of Pathology, Unity Hospital Laboratory Medical Director, AMC Crossroads Chaska and AMC Crossroads Dean Lakes Laboratory Testing for Celiac Disease Celiac
Coeliac disease. Do I have coeliac. disease? Diagnosis, monitoring & susceptibilty. Laboratory flowsheet included
 Laboratory flowsheet included I have coeliac disease. What monitoring tests should be performed? Do I have coeliac disease? Are either of our children susceptible to coeliac disease? Monitoring tests Diagnostic
Laboratory flowsheet included I have coeliac disease. What monitoring tests should be performed? Do I have coeliac disease? Are either of our children susceptible to coeliac disease? Monitoring tests Diagnostic
Disclosures GLUTEN RELATED DISORDERS CELIAC DISEASE UPDATE OR GLUTEN RELATED DISORDERS 6/9/2015
 Disclosures CELIAC DISEASE UPDATE OR GLUTEN RELATED DISORDERS 2015 Scientific Advisory Board: Alvine Pharmaceuticals, Alba Therapeutics, ImmunsanT Peter HR Green MD Columbia University New York, NY GLUTEN
Disclosures CELIAC DISEASE UPDATE OR GLUTEN RELATED DISORDERS 2015 Scientific Advisory Board: Alvine Pharmaceuticals, Alba Therapeutics, ImmunsanT Peter HR Green MD Columbia University New York, NY GLUTEN
TEST BULLETIN SUMMARY
 March 2018 Dear Healthcare Provider, The information contained here may be very important to your practice. Please take a moment to review this document. CHLAMYDIA/GONORRHEA SPECIMEN COLLECTION UPDATE
March 2018 Dear Healthcare Provider, The information contained here may be very important to your practice. Please take a moment to review this document. CHLAMYDIA/GONORRHEA SPECIMEN COLLECTION UPDATE
Primary Care Update January 26 & 27, 2017 Celiac Disease: Concepts & Conundrums
 Primary Care Update January 26 & 27, 2017 Celiac Disease: Concepts & Conundrums Alia Hasham, MD Assistant Professor Division of Gastroenterology, Hepatology & Nutrition What is the Preferred Initial Test
Primary Care Update January 26 & 27, 2017 Celiac Disease: Concepts & Conundrums Alia Hasham, MD Assistant Professor Division of Gastroenterology, Hepatology & Nutrition What is the Preferred Initial Test
Celiac & Gluten Sensitivity; serum
 TEST NAME: Celiac & Gluten Sensitivity (Serum) Celiac & Gluten Sensitivity; serum ANTIBODIES REFERENCE RESULT/UNIT INTERVAL NEG WEAK POS POSITIVE Tissue Transglutaminase (ttg) IgA 1420 U < 20.0 Tissue
TEST NAME: Celiac & Gluten Sensitivity (Serum) Celiac & Gluten Sensitivity; serum ANTIBODIES REFERENCE RESULT/UNIT INTERVAL NEG WEAK POS POSITIVE Tissue Transglutaminase (ttg) IgA 1420 U < 20.0 Tissue
Diseases of the gastrointestinal system Dr H Awad Lecture 5: diseases of the small intestine
 Diseases of the gastrointestinal system 2018 Dr H Awad Lecture 5: diseases of the small intestine Small intestinal villi Small intestinal villi -Villi are tall, finger like mucosal projections, found
Diseases of the gastrointestinal system 2018 Dr H Awad Lecture 5: diseases of the small intestine Small intestinal villi Small intestinal villi -Villi are tall, finger like mucosal projections, found
Is It Celiac Disease or Gluten Sensitivity?
 Is It Celiac Disease or Gluten Sensitivity? Mark T. DeMeo MD, FACG Rush University Med Center Case Study 35 y/o female Complains of diarrhea, bloating, arthralgias, and foggy mentation Cousin with celiac
Is It Celiac Disease or Gluten Sensitivity? Mark T. DeMeo MD, FACG Rush University Med Center Case Study 35 y/o female Complains of diarrhea, bloating, arthralgias, and foggy mentation Cousin with celiac
BIOPSY AVOIDANCE IN CHILDREN: THE EVIDENCE
 BIOPSY AVOIDANCE IN CHILDREN: THE EVIDENCE Steffen Husby Hans Christian Andersen Children s Hospital Odense University Hospital DK-5000 Odense C, Denmark Agenda Background Algorithm Symptoms HLA Antibodies
BIOPSY AVOIDANCE IN CHILDREN: THE EVIDENCE Steffen Husby Hans Christian Andersen Children s Hospital Odense University Hospital DK-5000 Odense C, Denmark Agenda Background Algorithm Symptoms HLA Antibodies
Living with Coeliac Disease Information & Support is key
 Living with Coeliac Disease Information & Support is key Mary Twohig Chairperson Coeliac Society of Ireland What is Coeliac Disease? LIVING WITH COELIAC DISEASE Fact Not Fad Auto immune disease - the body
Living with Coeliac Disease Information & Support is key Mary Twohig Chairperson Coeliac Society of Ireland What is Coeliac Disease? LIVING WITH COELIAC DISEASE Fact Not Fad Auto immune disease - the body
Epidemiology. The old Celiac Disease Epidemiology:
 Epidemiology 1 1 Epidemiology The old Celiac Disease Epidemiology: A rare disorder typical of infancy Wide incidence fluctuates in space (1/400 Ireland to 1/10000 Denmark) and in time A disease of essentially
Epidemiology 1 1 Epidemiology The old Celiac Disease Epidemiology: A rare disorder typical of infancy Wide incidence fluctuates in space (1/400 Ireland to 1/10000 Denmark) and in time A disease of essentially
Gluten sensitivity in Multiple Sclerosis Experimental myth or clinical truth?
 Gluten sensitivity in Multiple Sclerosis Experimental myth or clinical truth? Annals of the New York Academy of Sciences, Vol 1173, Issue 1, page 44, Issue published online 3 Sep 2009. Dana Ben-Ami Shor,
Gluten sensitivity in Multiple Sclerosis Experimental myth or clinical truth? Annals of the New York Academy of Sciences, Vol 1173, Issue 1, page 44, Issue published online 3 Sep 2009. Dana Ben-Ami Shor,
Evidence Based Guideline
 Evidence Based Guideline Serologic Diagnosis of Celiac Disease File Name: Origination: Last CAP Review: Next CAP Review: Last Review: serologic_diagnosis_of_celiac_disease 4/2012 Description of Procedure
Evidence Based Guideline Serologic Diagnosis of Celiac Disease File Name: Origination: Last CAP Review: Next CAP Review: Last Review: serologic_diagnosis_of_celiac_disease 4/2012 Description of Procedure
CONTEMPORARY CONCEPT ON BASIC APSECTS OF GLUTEN-SENSITIVE ENTEROPATHY IN ELDERLY PATIENTS
 VIII, 2014, 1 33. 1,. 2,. - 1,. 1. 3 1,., 2,., 3, CONTEMPORARY CONCEPT ON BASIC APSECTS OF GLUTEN-SENSITIVE ENTEROPATHY IN ELDERLY PATIENTS Ts. Velikova 1, Z. Spassova 2,. Ivanova-Todorova 1, D. Kyurkchiev
VIII, 2014, 1 33. 1,. 2,. - 1,. 1. 3 1,., 2,., 3, CONTEMPORARY CONCEPT ON BASIC APSECTS OF GLUTEN-SENSITIVE ENTEROPATHY IN ELDERLY PATIENTS Ts. Velikova 1, Z. Spassova 2,. Ivanova-Todorova 1, D. Kyurkchiev
Gluten-Free China Gastro Q&A
 Gluten-Free China Gastro Q&A Akiko Natalie Tomonari MD akiko.tomonari@parkway.cn Gastroenterology Specialist ParkwayHealth Introduction (of myself) Born in Japan, Raised in Maryland, USA Graduated from
Gluten-Free China Gastro Q&A Akiko Natalie Tomonari MD akiko.tomonari@parkway.cn Gastroenterology Specialist ParkwayHealth Introduction (of myself) Born in Japan, Raised in Maryland, USA Graduated from
Am I a Silly Yak? Laura Zakowski, MD. No financial disclosures
 Am I a Silly Yak? Laura Zakowski, MD No financial disclosures Patient NP 21 year old male with chronic headaches for 6 years extensively evaluated and treated Acupuncturist suggests testing for celiac
Am I a Silly Yak? Laura Zakowski, MD No financial disclosures Patient NP 21 year old male with chronic headaches for 6 years extensively evaluated and treated Acupuncturist suggests testing for celiac
QUANTA Lite TM Gliadin IgG II For In Vitro Diagnostic Use CLIA Complexity: High
 QUANTA Lite TM Gliadin IgG II 704520 For In Vitro Diagnostic Use CLIA Complexity: High Intended Use QUANTA Lite TM Gliadin IgG II is an enzyme-linked immunosorbent assay (ELISA) for the semi-quantitative
QUANTA Lite TM Gliadin IgG II 704520 For In Vitro Diagnostic Use CLIA Complexity: High Intended Use QUANTA Lite TM Gliadin IgG II is an enzyme-linked immunosorbent assay (ELISA) for the semi-quantitative
Meredythe A. McNally, M.D. Gastroenterology Associates of Cleveland Beachwood, OH
 Meredythe A. McNally, M.D. Gastroenterology Associates of Cleveland Beachwood, OH Case in point 42 year old woman with bloating, gas, intermittent diarrhea alternating with constipation, told she has IBS
Meredythe A. McNally, M.D. Gastroenterology Associates of Cleveland Beachwood, OH Case in point 42 year old woman with bloating, gas, intermittent diarrhea alternating with constipation, told she has IBS
CELIAC DISEASE - GENERAL AND LABORATORY ASPECTS Prof. Xavier Bossuyt, Ph.D. Laboratory Medicine, Immunology, University Hospital Leuven, Belgium
 CELIAC DISEASE - GENERAL AND LABORATORY ASPECTS Prof. Xavier Bossuyt, Ph.D. Laboratory Medicine, Immunology, University Hospital Leuven, Belgium 5.1 Introduction Celiac disease is a chronic immune-mediated
CELIAC DISEASE - GENERAL AND LABORATORY ASPECTS Prof. Xavier Bossuyt, Ph.D. Laboratory Medicine, Immunology, University Hospital Leuven, Belgium 5.1 Introduction Celiac disease is a chronic immune-mediated
QUANTA Lite TM h-ttg Screen For In Vitro Diagnostic Use CLIA Complexity: High
 QUANTA Lite TM h-ttg Screen 704570 For In Vitro Diagnostic Use CLIA Complexity: High Intended Use QUANTA Lite TM h-ttg Screen is an enzyme-linked immunosorbent assay (ELISA) for the semi-quantitative detection
QUANTA Lite TM h-ttg Screen 704570 For In Vitro Diagnostic Use CLIA Complexity: High Intended Use QUANTA Lite TM h-ttg Screen is an enzyme-linked immunosorbent assay (ELISA) for the semi-quantitative detection
The lab is open, the tests are available. Read on for much more information.
 From: *Dr. Tom O'Bryan * thedr.com Subject: The Tests That We've Been Waiting For ~ Gluten Sensitivity Related Testing Reply: karen@thedr.com Having trouble viewing this email? Click
From: *Dr. Tom O'Bryan * thedr.com Subject: The Tests That We've Been Waiting For ~ Gluten Sensitivity Related Testing Reply: karen@thedr.com Having trouble viewing this email? Click
Challenges in Celiac Disease. Adam Stein, MD Director of Nutrition Support Northwestern University Feinberg School of Medicine
 Challenges in Celiac Disease Adam Stein, MD Director of Nutrition Support Northwestern University Feinberg School of Medicine Disclosures None Overview Celiac disease Cases Celiac disease Inappropriate
Challenges in Celiac Disease Adam Stein, MD Director of Nutrition Support Northwestern University Feinberg School of Medicine Disclosures None Overview Celiac disease Cases Celiac disease Inappropriate
Baboons Affected by Hereditary Chronic Diarrhea as a Possible Non-Human Primate Model of Celiac Disease
 Baboons Affected by Hereditary Chronic Diarrhea as a Possible Non-Human Primate Model of Celiac Disease Debby Kryszak 1, Henry McGill 2, Michelle Leland 2,, Alessio Fasano 1 1. Center for Celiac Research,
Baboons Affected by Hereditary Chronic Diarrhea as a Possible Non-Human Primate Model of Celiac Disease Debby Kryszak 1, Henry McGill 2, Michelle Leland 2,, Alessio Fasano 1 1. Center for Celiac Research,
OHTAC Recommendation
 OHTAC Recommendation Clinical Utility of Serologic Testing for Celiac Disease in Ontario Presented to the Ontario Health Technology Advisory Committee in April and October, 2010 December 2010 Background
OHTAC Recommendation Clinical Utility of Serologic Testing for Celiac Disease in Ontario Presented to the Ontario Health Technology Advisory Committee in April and October, 2010 December 2010 Background
Update on Celiac Disease: New Standards and New Tests
 IMPROVING PATIENT CARE THROUGH ESOTERIC LABORATORY TESTING JUNE 2008 Update on Celiac Disease: New Standards and New Tests The National Institutes of Health (NIH) has reported that as many as 1% (3,000,000)
IMPROVING PATIENT CARE THROUGH ESOTERIC LABORATORY TESTING JUNE 2008 Update on Celiac Disease: New Standards and New Tests The National Institutes of Health (NIH) has reported that as many as 1% (3,000,000)
ImuPro shows you the way to the right food for you. And your path for better health.
 Your personal ImuPro Screen + documents Sample ID: 33333 Dear, With this letter, you will receive the ImuPro result for your personal IgG food allergy test. This laboratory report contains your results
Your personal ImuPro Screen + documents Sample ID: 33333 Dear, With this letter, you will receive the ImuPro result for your personal IgG food allergy test. This laboratory report contains your results
ab Anti-Deamidated Gliadin Peptide (DGP) IgG ELISA Kit
 ab178617 Anti-Deamidated Gliadin Peptide (DGP) IgG ELISA Kit Instructions for Use For the quantitative measurement of IgG class antibodies against Deamidated Gliadin Peptide (DGP) in Human serum and plasma.
ab178617 Anti-Deamidated Gliadin Peptide (DGP) IgG ELISA Kit Instructions for Use For the quantitative measurement of IgG class antibodies against Deamidated Gliadin Peptide (DGP) in Human serum and plasma.
Follow-up Management of Patients with Celiac Disease: Resource for Health Professionals
 Follow-up Management of Patients with Celiac Disease: Resource for Health Professionals Jocelyn Silvester, MD PhD FRCPC April 27, 2017 Research grants Disclosures Canadian Institutes of Health Research
Follow-up Management of Patients with Celiac Disease: Resource for Health Professionals Jocelyn Silvester, MD PhD FRCPC April 27, 2017 Research grants Disclosures Canadian Institutes of Health Research
Peter HR Green MD. Columbia University New York, NY
 CELIAC DISEASE, 2008 Peter HR Green MD Celiac Disease Center Columbia University New York, NY pg11@columbia.edu DIAGNOSIS OF CELIAC DISEASE Presence of consistent pathology and response to a gluten-free
CELIAC DISEASE, 2008 Peter HR Green MD Celiac Disease Center Columbia University New York, NY pg11@columbia.edu DIAGNOSIS OF CELIAC DISEASE Presence of consistent pathology and response to a gluten-free
 27027 Tourney Road Valencia, CA 91355 800 421 7110 www.specialtylabs.com Test Updates May 27, 2009 Dear Colleague: Specialty Laboratories is pleased to announce the immediate availability of a new molecular
27027 Tourney Road Valencia, CA 91355 800 421 7110 www.specialtylabs.com Test Updates May 27, 2009 Dear Colleague: Specialty Laboratories is pleased to announce the immediate availability of a new molecular
Gluten Sensitivity Fact from Myth. Disclosures OBJECTIVES 18/09/2013. Justine Turner MD PhD University of Alberta. None Relevant
 Gluten Sensitivity Fact from Myth Justine Turner MD PhD University of Alberta Disclosures None Relevant OBJECTIVES Understand the spectrum of gluten disorders Develop a diagnostic algorithm for gluten
Gluten Sensitivity Fact from Myth Justine Turner MD PhD University of Alberta Disclosures None Relevant OBJECTIVES Understand the spectrum of gluten disorders Develop a diagnostic algorithm for gluten
Immuno Bloodprint Reactive Foods:
 Patient: Sample Patient Physician: Sample Physician Immuno Bloodprint Reactive Foods: Bean, Kidney (+2) Milk, Goat s (+1) Sesame (+1) Bean, Pinto (+1) Mushroom (+1) Soybean (+1) Cheese (+1) Oat (+1) Spinach
Patient: Sample Patient Physician: Sample Physician Immuno Bloodprint Reactive Foods: Bean, Kidney (+2) Milk, Goat s (+1) Sesame (+1) Bean, Pinto (+1) Mushroom (+1) Soybean (+1) Cheese (+1) Oat (+1) Spinach
Celiac Disease: The Future. Alessio Fasano, M.D. Mucosal Biology Research Center University of Maryland School of Medicine
 Celiac Disease: The Future Alessio Fasano, M.D. Mucosal Biology Research Center University of Maryland School of Medicine Normal small bowel Celiac disease Gluten Gluten-free diet Treatment Only treatment
Celiac Disease: The Future Alessio Fasano, M.D. Mucosal Biology Research Center University of Maryland School of Medicine Normal small bowel Celiac disease Gluten Gluten-free diet Treatment Only treatment
Sunanda Kane, MD, MSPH, FACG, FACP, AGAF Associate Professor of Medicine Mayo Clinic
 Serum Markers: What, Who, When and Why? Sunanda Kane, MD, MSPH, FACG, FACP, AGAF Associate Professor of Medicine Mayo Clinic Crohn s Disease: Microbial Antibodies ASCA Anti-I2 Anti-OmpC Bir1 Flagellin
Serum Markers: What, Who, When and Why? Sunanda Kane, MD, MSPH, FACG, FACP, AGAF Associate Professor of Medicine Mayo Clinic Crohn s Disease: Microbial Antibodies ASCA Anti-I2 Anti-OmpC Bir1 Flagellin
Celiac Disease and Non Celiac Gluten Sensitivity. John R Cangemi, MD Mayo Clinic Florida
 Celiac Disease and Non Celiac Gluten Sensitivity John R Cangemi, MD Mayo Clinic Florida DISCLOSURE Commercial Interest None Off Label Usage None Learning Objectives Review the clinical presentation of
Celiac Disease and Non Celiac Gluten Sensitivity John R Cangemi, MD Mayo Clinic Florida DISCLOSURE Commercial Interest None Off Label Usage None Learning Objectives Review the clinical presentation of
Spectrum of Gluten Disorders
 Food Intolerance:Celiac Disease and Gluten Sensitivity-A Guide for Healthy Lifestyles Ellen Karlin 2018 Spectrum of Gluten Disorders Wheat allergy - prevalence 3-8 % (up to 3 years old) Non-celiac gluten
Food Intolerance:Celiac Disease and Gluten Sensitivity-A Guide for Healthy Lifestyles Ellen Karlin 2018 Spectrum of Gluten Disorders Wheat allergy - prevalence 3-8 % (up to 3 years old) Non-celiac gluten
Improving allergy outcomes. IgE and IgG 4 food serology in a Gastroenterology Practice. Jay Weiss, Ph.D and Gary Kitos, Ph.D., H.C.L.D.
 Improving allergy outcomes IgE and IgG 4 food serology in a Gastroenterology Practice Jay Weiss, Ph.D and Gary Kitos, Ph.D., H.C.L.D. IgE and IgG4 food serology in a gastroenterology practice The following
Improving allergy outcomes IgE and IgG 4 food serology in a Gastroenterology Practice Jay Weiss, Ph.D and Gary Kitos, Ph.D., H.C.L.D. IgE and IgG4 food serology in a gastroenterology practice The following
Antibodies Against Synthetic Deamidated Gliadin Peptides and Tissue Transglutaminase for the Identification of Childhood Celiac Disease
 CLINICAL GASTROENTEROLOGY AND HEPATOLOGY 2007;5:1276 1281 Antibodies Against Synthetic Deamidated Gliadin Peptides and Tissue Transglutaminase for the Identification of Childhood Celiac Disease DANIEL
CLINICAL GASTROENTEROLOGY AND HEPATOLOGY 2007;5:1276 1281 Antibodies Against Synthetic Deamidated Gliadin Peptides and Tissue Transglutaminase for the Identification of Childhood Celiac Disease DANIEL
Diet Isn t Working, We Need to Do Something Else
 Diet Isn t Working, We Need to Do Something Else Ciarán P Kelly, MD Celiac Center Beth Israel Deaconess Medical Center & Celiac Program Harvard Medical School Boston Gluten Free Diet (GFD) Very good but
Diet Isn t Working, We Need to Do Something Else Ciarán P Kelly, MD Celiac Center Beth Israel Deaconess Medical Center & Celiac Program Harvard Medical School Boston Gluten Free Diet (GFD) Very good but
Clinical Policy Title: Celiac disease diagnostic testing
 Clinical Policy Title: Celiac disease diagnostic testing Clinical Policy Number: CCP.1049 Effective Date: December 1, 2013 Initial Review Date: August 21, 2013 Most Recent Review Date: August 7, 2018 Next
Clinical Policy Title: Celiac disease diagnostic testing Clinical Policy Number: CCP.1049 Effective Date: December 1, 2013 Initial Review Date: August 21, 2013 Most Recent Review Date: August 7, 2018 Next
Pediatric Food Allergies: Physician and Parent. Robert Anderson MD Rachel Anderson Syracuse, NY March 3, 2018
 Pediatric Food Allergies: Physician and Parent Robert Anderson MD Rachel Anderson Syracuse, NY March 3, 2018 Learning Objectives Identify risk factors for food allergies Identify clinical manifestations
Pediatric Food Allergies: Physician and Parent Robert Anderson MD Rachel Anderson Syracuse, NY March 3, 2018 Learning Objectives Identify risk factors for food allergies Identify clinical manifestations
International Journal of Health Sciences and Research ISSN:
 International Journal of Health Sciences and Research www.ijhsr.org ISSN: 2249-9571 Original Research Article Role of Blood TTG and Small Intestine Biopsy in Diagnosis of Celiac Disease Anil Batta Professor,
International Journal of Health Sciences and Research www.ijhsr.org ISSN: 2249-9571 Original Research Article Role of Blood TTG and Small Intestine Biopsy in Diagnosis of Celiac Disease Anil Batta Professor,
Celiac Disease For Dummies By Sheila Crowe, Ian Blumer READ ONLINE
 Celiac Disease For Dummies By Sheila Crowe, Ian Blumer READ ONLINE Celiac disease definition, a hereditary digestive disorder involving intolerance to gluten, usually occurring in young children, characterized
Celiac Disease For Dummies By Sheila Crowe, Ian Blumer READ ONLINE Celiac disease definition, a hereditary digestive disorder involving intolerance to gluten, usually occurring in young children, characterized
EAT ACCORDING TO YOUR GENES. NGx-Gluten TM. Personalized Nutrition Report
 EAT ACCORDING TO YOUR GENES NGx-Gluten TM Personalized Nutrition Report Introduction Hello Caroline: Nutrigenomix is pleased to provide you with your NGx-Gluten TM Personalized Nutrition Report based on
EAT ACCORDING TO YOUR GENES NGx-Gluten TM Personalized Nutrition Report Introduction Hello Caroline: Nutrigenomix is pleased to provide you with your NGx-Gluten TM Personalized Nutrition Report based on
ASCA IgG/IgA ELISA Kit
 ASCA IgG/IgA ELISA Kit Catalog Number KA1270 96 assays Version: 02 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle of the Assay...
ASCA IgG/IgA ELISA Kit Catalog Number KA1270 96 assays Version: 02 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle of the Assay...
FOOD ALLERGY PANEL TESTING UPDATE AND ALLERGY REFLEX TESTING
 October 2015 Dear Healthcare Provider, The information contained here may be very important to your practice. Please take a moment to review this document. Details are on the included pages. FOOD ALLERGY
October 2015 Dear Healthcare Provider, The information contained here may be very important to your practice. Please take a moment to review this document. Details are on the included pages. FOOD ALLERGY
By Mathew P. Estey, PhD, FCACB; and Vilte E. Barakauskas, PhD, DABCC, FCACB
 1 of 5 2015-07-10 11:15 AM Evolution of Celiac Disease Testing The laboratory is challenged to provide guidance on test ordering and interpretation while ensuring accurate performance and appropriate test
1 of 5 2015-07-10 11:15 AM Evolution of Celiac Disease Testing The laboratory is challenged to provide guidance on test ordering and interpretation while ensuring accurate performance and appropriate test
Gliadin antibody detection in gluten
 The Ulster Medical Journal, Volume 55, No. 2, pp. 160-164, October 1986. Gliadin antibody detection in gluten enteropathy R G P Watson, S A McMillan, Clare Dolan, Cliona O'Farrelly, R J G Cuthbert, Margaret
The Ulster Medical Journal, Volume 55, No. 2, pp. 160-164, October 1986. Gliadin antibody detection in gluten enteropathy R G P Watson, S A McMillan, Clare Dolan, Cliona O'Farrelly, R J G Cuthbert, Margaret
Name of Policy: Human Leukocyte Antigen (HLA) Testing for Celiac Disease
 Name of Policy: Human Leukocyte Antigen (HLA) Testing for Celiac Disease Policy #: 545 Latest Review Date: June 2015 Category: Laboratory Policy Grade: B Background/Definitions: As a general rule, benefits
Name of Policy: Human Leukocyte Antigen (HLA) Testing for Celiac Disease Policy #: 545 Latest Review Date: June 2015 Category: Laboratory Policy Grade: B Background/Definitions: As a general rule, benefits
588-Complete Dietary Antigen Testing
 REPORT-1857 9 Dunwoody Park, Suite 121 Dunwoody, GA 3338 P: 678-736-6374 F: 77-674-171 Email: info@dunwoodylabs.com www.dunwoodylabs.com PATIENT INFO NAME: SAMPE PATIENT REQUISITION ID: 1857 SAMPE ID:
REPORT-1857 9 Dunwoody Park, Suite 121 Dunwoody, GA 3338 P: 678-736-6374 F: 77-674-171 Email: info@dunwoodylabs.com www.dunwoodylabs.com PATIENT INFO NAME: SAMPE PATIENT REQUISITION ID: 1857 SAMPE ID:
Name of Policy: Serologic Diagnosis of Celiac Disease
 Name of Policy: Serologic Diagnosis of Celiac Disease Policy #: 161 Latest Review Date: September 2013 Category: Laboratory Policy Grade: A Background/Definitions: As a general rule, benefits are payable
Name of Policy: Serologic Diagnosis of Celiac Disease Policy #: 161 Latest Review Date: September 2013 Category: Laboratory Policy Grade: A Background/Definitions: As a general rule, benefits are payable
Celiac disease (CD) is a gluten-sensitive enteropathy with. Comparative Usefulness of Deamidated Gliadin Antibodies in the Diagnosis of Celiac Disease
 CLINICAL GASTROENTEROLOGY AND HEPATOLOGY 2008;6:426 432 Comparative Usefulness of Deamidated Gliadin Antibodies in the Diagnosis of Celiac Disease SHADI RASHTAK,* MICHAEL W. ETTORE, HENRY A. HOMBURGER,
CLINICAL GASTROENTEROLOGY AND HEPATOLOGY 2008;6:426 432 Comparative Usefulness of Deamidated Gliadin Antibodies in the Diagnosis of Celiac Disease SHADI RASHTAK,* MICHAEL W. ETTORE, HENRY A. HOMBURGER,
Celiac Disease and Immunoglobulin A Deficiency: How Effective Are the Serological Methods of Diagnosis?
 CLINICAL AND DIAGNOSTIC LABORATORY IMMUNOLOGY, Nov. 2002, p. 1295 1300 Vol. 9, No. 6 1071-412X/02/$04.00 0 DOI: 10.1128/CDLI.9.6.1295 1300.2002 Copyright 2002, American Society for Microbiology. All Rights
CLINICAL AND DIAGNOSTIC LABORATORY IMMUNOLOGY, Nov. 2002, p. 1295 1300 Vol. 9, No. 6 1071-412X/02/$04.00 0 DOI: 10.1128/CDLI.9.6.1295 1300.2002 Copyright 2002, American Society for Microbiology. All Rights
Rebecca Rovay-Hazelton Licensed Nutritionist, Functional Diagnostic Nutritionist
 Rebecca Rovay-Hazelton Licensed Nutritionist, Functional Diagnostic Nutritionist Section 1: What is gluten? Foods containing gluten Section 2: What is gluten intolerance? Section 3: Testing for gluten
Rebecca Rovay-Hazelton Licensed Nutritionist, Functional Diagnostic Nutritionist Section 1: What is gluten? Foods containing gluten Section 2: What is gluten intolerance? Section 3: Testing for gluten
Original Policy Date
 MP 2.04.21 Serologic Diagnosis of Celiac Disease Medical Policy Section Medicine Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Reviewed with literature search/12:2013 Return to Medical
MP 2.04.21 Serologic Diagnosis of Celiac Disease Medical Policy Section Medicine Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Reviewed with literature search/12:2013 Return to Medical
MULTIPLE FOOD IMMUNE REACTIVITY SCREEN
 10 MULTIPLE FOOD IMMUNE REACTIVITY SCREEN 180 Real-World Food Antigens COOKED RAW MODIFIED ONE PANEL Modified Cooked Cooked Raw Evaluate immune reactions to foods, raw and/or modified, food enzymes, lectins
10 MULTIPLE FOOD IMMUNE REACTIVITY SCREEN 180 Real-World Food Antigens COOKED RAW MODIFIED ONE PANEL Modified Cooked Cooked Raw Evaluate immune reactions to foods, raw and/or modified, food enzymes, lectins
23 Studies on Low-Carb and Low-Fat Diets Time to Retire the Fad
 23 Studies on Low-Carb and Low-Fat Diets Time to Retire the Fad Kris Gunnars, BSc Few things have been debated as much as carbohydrates vs fat. Some believe that increased fat in the diet is a leading
23 Studies on Low-Carb and Low-Fat Diets Time to Retire the Fad Kris Gunnars, BSc Few things have been debated as much as carbohydrates vs fat. Some believe that increased fat in the diet is a leading
Celiac Disease Ce. Celiac Disease. Barry Z. Hirsch, M.D. Baystate Pediatric Gastroenterology and Nutrition. baystatehealth.org/bch
 Celiac Disease Ce Celiac Disease Barry Z. Hirsch, M.D. Baystate Pediatric Gastroenterology and Nutrition baystatehealth.org/bch Autoimmune Disease Inappropriate inflammation 1 1/21/15 Celiac Disease Classic
Celiac Disease Ce Celiac Disease Barry Z. Hirsch, M.D. Baystate Pediatric Gastroenterology and Nutrition baystatehealth.org/bch Autoimmune Disease Inappropriate inflammation 1 1/21/15 Celiac Disease Classic
New Insights on Gluten Sensitivity
 New Insights on Gluten Sensitivity Sheila E. Crowe, MD, FRCPC, FACP, FACG, AGAF Department of Medicine University of California, San Diego Page 1 1 low fat diet low carb diet gluten free diet low fat diet
New Insights on Gluten Sensitivity Sheila E. Crowe, MD, FRCPC, FACP, FACG, AGAF Department of Medicine University of California, San Diego Page 1 1 low fat diet low carb diet gluten free diet low fat diet
Sheila E. Crowe, MD, FACG
 1A: Upper Gut Celiac Disease: When to Look and How? Sheila E. Crowe, MD, FACG Learning Objectives At the end of this presentation, the successful learner should be able to: Identify the many groups of
1A: Upper Gut Celiac Disease: When to Look and How? Sheila E. Crowe, MD, FACG Learning Objectives At the end of this presentation, the successful learner should be able to: Identify the many groups of
*Please see amendment for Pennsylvania Medicaid at the end
 1 of 28 Number: 0561 Policy *Please see amendment for Pennsylvania Medicaid at the end of this CPB. I. Aetna considers serological testing of IgA anti human tissue transglutaminase (TTG) antibodies, IgG
1 of 28 Number: 0561 Policy *Please see amendment for Pennsylvania Medicaid at the end of this CPB. I. Aetna considers serological testing of IgA anti human tissue transglutaminase (TTG) antibodies, IgG
Celiac Disease. Etiology. Food Intolerance:Celiac Disease and Gluten Sensitivity-A Guide for Healthy Lifestyles
 Food Intolerance:Celiac Disease and Gluten Sensitivity-A Guide for Healthy Lifestyles Ellen Karlin 2017 Celiac Disease World s most common genetic food disorder Rising prevalence - over past 5 decades,
Food Intolerance:Celiac Disease and Gluten Sensitivity-A Guide for Healthy Lifestyles Ellen Karlin 2017 Celiac Disease World s most common genetic food disorder Rising prevalence - over past 5 decades,
Celiac Disease: The Quintessential Autoimmune Disease Ivor D. Hill, MB, ChB, MD.
 Celiac Disease: The Quintessential Autoimmune Disease Ivor D. Hill, MB, ChB, MD..... Celiac Disease Autoimmune Diseases What are they? How do you get them? Why does it matter? Celiac Disease Autoimmune
Celiac Disease: The Quintessential Autoimmune Disease Ivor D. Hill, MB, ChB, MD..... Celiac Disease Autoimmune Diseases What are they? How do you get them? Why does it matter? Celiac Disease Autoimmune
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Serologic Diagnosis of Celiac Disease Page 1 of 14 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Serologic Diagnosis of Celiac Disease Professional Institutional
Serologic Diagnosis of Celiac Disease Page 1 of 14 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Serologic Diagnosis of Celiac Disease Professional Institutional
CELIAC SPRUE. What Happens With Celiac Disease
 CELIAC SPRUE Celiac Disease (CD) is a lifelong, digestive disorder affecting children and adults. When people with CD eat foods that contain gluten, it creates an immune-mediated toxic reaction that causes
CELIAC SPRUE Celiac Disease (CD) is a lifelong, digestive disorder affecting children and adults. When people with CD eat foods that contain gluten, it creates an immune-mediated toxic reaction that causes
Celiac Disease 1/13/2016. Objectives. Question 1. Understand the plethora of conditions or symptoms that require testing for Celiac Disease (CD)
 Celiac Disease MONTE E. TROUTMAN, DO, FACOI JANUARY 6, 2016 Objectives Understand the plethora of conditions or symptoms that require testing for Celiac Disease (CD) Develop a knowledge of testing needed
Celiac Disease MONTE E. TROUTMAN, DO, FACOI JANUARY 6, 2016 Objectives Understand the plethora of conditions or symptoms that require testing for Celiac Disease (CD) Develop a knowledge of testing needed
WHY IS THERE CONTROVERSY ABOUT FOOD ALLERGY AND ECZEMA. Food Allergies and Eczema: Facts and Fallacies
 Food Allergies and Eczema: Facts and Fallacies Lawrence F. Eichenfield,, M.D. Professor of Clinical Pediatrics and Medicine (Dermatology) University of California, San Diego Rady Children s s Hospital,
Food Allergies and Eczema: Facts and Fallacies Lawrence F. Eichenfield,, M.D. Professor of Clinical Pediatrics and Medicine (Dermatology) University of California, San Diego Rady Children s s Hospital,
Celiac disease is a unique disorder that is both a food
 GASTROENTEROLOGY 2006;131:1981 2002 American Gastroenterological Association () Institute Technical Review on the Diagnosis and Management of Celiac Disease This technical review addresses the state of
GASTROENTEROLOGY 2006;131:1981 2002 American Gastroenterological Association () Institute Technical Review on the Diagnosis and Management of Celiac Disease This technical review addresses the state of
History of Food Allergies
 Grand Valley State University From the SelectedWorks of Jody L Vogelzang PhD, RDN, FAND, CHES Spring 2013 History of Food Allergies Jody L Vogelzang, PhD, RDN, FAND, CHES, Grand Valley State University
Grand Valley State University From the SelectedWorks of Jody L Vogelzang PhD, RDN, FAND, CHES Spring 2013 History of Food Allergies Jody L Vogelzang, PhD, RDN, FAND, CHES, Grand Valley State University
prevalence 181 Atopy patch test, see Patch test
 Subject Index AD, see Atopic dermatitis Adrenaline, anaphylaxis management 99 101, 194, 195 Adverse food reaction definition 4 nonallergic reactions 6, 9 Allergen Nomenclature database 20, 21 Allergen
Subject Index AD, see Atopic dermatitis Adrenaline, anaphylaxis management 99 101, 194, 195 Adverse food reaction definition 4 nonallergic reactions 6, 9 Allergen Nomenclature database 20, 21 Allergen
INTEGRATIVE MEDICINE
 TEST PATIENT TEST PHYSICIAN DR JOHN DOE 6DPSOH 7HVW 1DPH Sex : ) 111 CLINIC ST5((7 DDWH Collected : 00-00-0000 &/,1,& 68%85% 9,& 111 7(67 ROAD TEST SUBURB /AB,': 00000000 UR#:0000000 IgA P: 1300 688 522
TEST PATIENT TEST PHYSICIAN DR JOHN DOE 6DPSOH 7HVW 1DPH Sex : ) 111 CLINIC ST5((7 DDWH Collected : 00-00-0000 &/,1,& 68%85% 9,& 111 7(67 ROAD TEST SUBURB /AB,': 00000000 UR#:0000000 IgA P: 1300 688 522
LORAIN COUNTY COMMISSIONERS
 LORAIN COUNTY COMMISSIONERS LabInfo Newsletter Supplemental Edition Volume 1, Number 2 September-December, 2014 This newsletter is provided by the Lorain County Crime/Drug Lab discussing technical information
LORAIN COUNTY COMMISSIONERS LabInfo Newsletter Supplemental Edition Volume 1, Number 2 September-December, 2014 This newsletter is provided by the Lorain County Crime/Drug Lab discussing technical information
DDW WRAP-UP 2012 CELIAC DISEASE. Anju Sidhu MD University of Louisville Gastroenterology, Hepatology and Nutrition June 21, 2012
 DDW WRAP-UP 2012 CELIAC DISEASE Anju Sidhu MD University of Louisville Gastroenterology, Hepatology and Nutrition June 21, 2012 OVERVIEW Definition Susceptibility The Changing Clinical Presentation Medical
DDW WRAP-UP 2012 CELIAC DISEASE Anju Sidhu MD University of Louisville Gastroenterology, Hepatology and Nutrition June 21, 2012 OVERVIEW Definition Susceptibility The Changing Clinical Presentation Medical
Comparison of Commercially Available Serologic Kits for the Detection of Celiac Disease
 ORIGINAL ARTICLE Comparison of Commercially Available Serologic Kits for the Detection of Celiac Disease Afzal J. Naiyer, MD, Lincoln Hernandez, MD, Edward J. Ciaccio, PhD, Konstantinos Papadakis, MD,
ORIGINAL ARTICLE Comparison of Commercially Available Serologic Kits for the Detection of Celiac Disease Afzal J. Naiyer, MD, Lincoln Hernandez, MD, Edward J. Ciaccio, PhD, Konstantinos Papadakis, MD,
The Clinical Response to Gluten Challenge: A Review of the Literature
 Nutrients 2013, 5, 4614-4641; doi:.3390/nu5114614 Review OPEN ACCESS nutrients ISSN 2072-6643 www.mdpi.com/journal/nutrients The Clinical Response to Gluten Challenge: A Review of the Literature Maaike
Nutrients 2013, 5, 4614-4641; doi:.3390/nu5114614 Review OPEN ACCESS nutrients ISSN 2072-6643 www.mdpi.com/journal/nutrients The Clinical Response to Gluten Challenge: A Review of the Literature Maaike
Functional Medicine Is the application of alternative holistic measures to show people how to reverse thyroid conditions, endocrine issues, hormone
 Functional Medicine Is the application of alternative holistic measures to show people how to reverse thyroid conditions, endocrine issues, hormone issues, fibromyalgia, autoimmunity diseases and the like.
Functional Medicine Is the application of alternative holistic measures to show people how to reverse thyroid conditions, endocrine issues, hormone issues, fibromyalgia, autoimmunity diseases and the like.
Slides and Resources.
 Update on Celiac Disease Douglas L. Seidner, MD, AGAF, FACG Director, Center for Human Nutrition Vanderbilt University As revised/retold by Edward Saltzman, MD Tufts University None Disclosures This ppt
Update on Celiac Disease Douglas L. Seidner, MD, AGAF, FACG Director, Center for Human Nutrition Vanderbilt University As revised/retold by Edward Saltzman, MD Tufts University None Disclosures This ppt
Utility in Clinical Practice of Immunoglobulin A Anti-Tissue Transglutaminase Antibody for the Diagnosis of Celiac Disease
 CLINICAL GASTROENTEROLOGY AND HEPATOLOGY 2006;4:726 730 Utility in Clinical Practice of Immunoglobulin A Anti-Tissue Transglutaminase Antibody for the Diagnosis of Celiac Disease JULIAN A. ABRAMS,* PARDEEP
CLINICAL GASTROENTEROLOGY AND HEPATOLOGY 2006;4:726 730 Utility in Clinical Practice of Immunoglobulin A Anti-Tissue Transglutaminase Antibody for the Diagnosis of Celiac Disease JULIAN A. ABRAMS,* PARDEEP
CELIAC DISEASE. Molly Jennings Deb McCafferty MS, RD
 CELIAC DISEASE Molly Jennings Deb McCafferty MS, RD WHAT IS CELIAC DISEASE? In short In this disease, exposure to gluten results in damge to the intestinal mucosa. Immune-mediated disorder Also known as
CELIAC DISEASE Molly Jennings Deb McCafferty MS, RD WHAT IS CELIAC DISEASE? In short In this disease, exposure to gluten results in damge to the intestinal mucosa. Immune-mediated disorder Also known as
What is celiac disease?
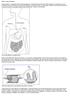 What is celiac disease? Celiac disease is a digestive disease that damages the small intestine and interferes with absorption of nutrients from food. People who have celiac disease cannot tolerate gluten,
What is celiac disease? Celiac disease is a digestive disease that damages the small intestine and interferes with absorption of nutrients from food. People who have celiac disease cannot tolerate gluten,
Clinical Policy: Celiac Disease Laboratory Testing Reference Number: CP.MP.HN255
 Clinical Policy: Reference Number: CP.MP.HN255 Effective Date: 02/06 Last Review Date: 7/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and
Clinical Policy: Reference Number: CP.MP.HN255 Effective Date: 02/06 Last Review Date: 7/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and
Saeeda Almarzooqi, 1 Ronald H. Houston, 2 and Vinay Prasad Introduction
 Pathology Research International Volume 2013, Article ID 602985, 5 pages http://dx.doi.org/10.1155/2013/602985 Clinical Study Utility of Tissue Transglutaminase Immunohistochemistry in Pediatric Duodenal
Pathology Research International Volume 2013, Article ID 602985, 5 pages http://dx.doi.org/10.1155/2013/602985 Clinical Study Utility of Tissue Transglutaminase Immunohistochemistry in Pediatric Duodenal
Frontiers in Food Allergy and Allergen Risk Assessment and Management. 19 April 2018, Madrid
 Frontiers in Food Allergy and Allergen Risk Assessment and Management 19 April 2018, Madrid Food allergy is becoming one of the serious problems of China's food safety and public health emergency. 7 Number
Frontiers in Food Allergy and Allergen Risk Assessment and Management 19 April 2018, Madrid Food allergy is becoming one of the serious problems of China's food safety and public health emergency. 7 Number
Alliance for Best Practice in Health Education
 Alliance for Best Practice in Health Education Objectives Following this program, participants will 1. List the clinical situations where celiac disease should be suspected 2. Distinguish between celiac
Alliance for Best Practice in Health Education Objectives Following this program, participants will 1. List the clinical situations where celiac disease should be suspected 2. Distinguish between celiac
Presentation and Evaluation of Celiac Disease
 Presentation and Evaluation of Celiac Disease C. CUFFARI, MD, FRCPC, FACG, AGAF The Johns Hopkins Hospital Baltimore MD. Main Points Celiac disease is not rare (1 in 100-300) It can present in many ways:
Presentation and Evaluation of Celiac Disease C. CUFFARI, MD, FRCPC, FACG, AGAF The Johns Hopkins Hospital Baltimore MD. Main Points Celiac disease is not rare (1 in 100-300) It can present in many ways:
GliaDea IgA ELISA. Gliadin IgA ELISA Assay Kit 1/7 Catalog Number: GDA31-K01. INTENDED USE
 INTENDED USE The Eagle Biosciences GliaDea IgA ELISA Assay Kit is used for the quantitative determination of IgA antibodies against deamidated gliadin in human serum or plasma for the diagnosis of celiac
INTENDED USE The Eagle Biosciences GliaDea IgA ELISA Assay Kit is used for the quantitative determination of IgA antibodies against deamidated gliadin in human serum or plasma for the diagnosis of celiac
Elements for a Public Summary
 VI.2 VI.2.1 Elements for a Public Summary Overview of disease epidemiology Acitretin Orifarm is indicated as treatment for severe skin problems where the skin has become thick and may be scaly. Acitretin
VI.2 VI.2.1 Elements for a Public Summary Overview of disease epidemiology Acitretin Orifarm is indicated as treatment for severe skin problems where the skin has become thick and may be scaly. Acitretin
Understanding Celiac Disease
 Understanding Celiac Disease Diagnostic Challenges Sheryl Pfeil, MD Professor of Clinical Medicine Division of Gastroenterology, Hepatology and Nutrition Department of Internal Medicine The Ohio State
Understanding Celiac Disease Diagnostic Challenges Sheryl Pfeil, MD Professor of Clinical Medicine Division of Gastroenterology, Hepatology and Nutrition Department of Internal Medicine The Ohio State
Immune mediated enteropathies. Aurora Tatu Bern 26/07/2017
 Immune mediated enteropathies Aurora Tatu Bern 26/07/2017 Definition/classification Systemic disease, mediated by antibodies, caracterised by histological changes of the small bowel Coeliac and noncoeliac
Immune mediated enteropathies Aurora Tatu Bern 26/07/2017 Definition/classification Systemic disease, mediated by antibodies, caracterised by histological changes of the small bowel Coeliac and noncoeliac
Understanding Celiac Disease
 Understanding Diagnostic Challenges Sheryl Pfeil, MD Professor of Clinical Medicine Division of Gastroenterology, Hepatology and Nutrition Department of Internal Medicine The Ohio State University Wexner
Understanding Diagnostic Challenges Sheryl Pfeil, MD Professor of Clinical Medicine Division of Gastroenterology, Hepatology and Nutrition Department of Internal Medicine The Ohio State University Wexner
The Significance of IgG Antibodies against Tissue Transglutaminase in Coeliac Disease
 Digital Comprehensive Summaries of Uppsala Dissertations from the Faculty of Medicine 307 The Significance of IgG Antibodies against Tissue Transglutaminase in Coeliac Disease INGRID DAHLBOM ACTA UNIVERSITATIS
Digital Comprehensive Summaries of Uppsala Dissertations from the Faculty of Medicine 307 The Significance of IgG Antibodies against Tissue Transglutaminase in Coeliac Disease INGRID DAHLBOM ACTA UNIVERSITATIS
Review Article. Anti-tissue transglutaminase antibodies and their role in the investigation of coeliac disease. Clinical background to coeliac disease
 Review Article Anti-tissue transglutaminase antibodies and their role in the investigation of coeliac disease PG Hill 1 and SA McMillan 2 Abstract Addresses 1 Department of Chemical Pathology, Haematology
Review Article Anti-tissue transglutaminase antibodies and their role in the investigation of coeliac disease PG Hill 1 and SA McMillan 2 Abstract Addresses 1 Department of Chemical Pathology, Haematology
