Diversity of Secondary Metabolites in the Genus Silene L. (Caryophyllaceae) Structures, Distribution, and Biological Properties
|
|
|
- Daniela Simpson
- 6 years ago
- Views:
Transcription
1 Diversity 2014, 6, ; doi: /d Review PEN ACCESS diversity ISSN Diversity of Secondary Metabolites in the Genus Silene L. (Caryophyllaceae) Structures, Distribution, and Biological Properties Nilufar Z. Mamadalieva 1, Rene Lafont 2 and Michael Wink 3, * Institute of the Chemistry of Plant Substances AS RUz, Mirzo Ulugbek Str. 77, Tashkent , Uzbekistan; nmamadalieva@yahoo.com Sorbonne Universités, Université Pierre et Marie Curie, IBPS-BISIPE, Case Courrier n 29, 7 Quai Saint Bernard F-75252, Paris cedex 05, France; rene.lafont@snv.jussieu.fr eidelberg University, Institute of Pharmacy and Molecular Biotechnology, Im Neuenheimer Feld 364, eidelberg, Germany * Author to whom correspondence should be addressed; wink@uni-heidelberg.de; Tel.: ; Fax: Received: 21 May 2014; in revised form: 26 June 2014 / Accepted: 1 July 2014 / Published: 11 July 2014 Abstract: The genus Silene (family Caryophyllaceae) comprises more than 700 species, which are widely distributed in temperate zones of the Northern emisphere, but are also present in Africa and have been introduced in other continents. Silene produces a high diversity of secondary metabolites and many of them show interesting biological and pharmacological activities. More than 450 compounds have been isolated; important classes include phytoecdysteroids (which mimic insect molting hormones), triterpene saponins (with detergent properties), volatiles, other terpenoids and phenolics. This review focusses on the phytochemical diversity, distribution of Silene secondary metabolites and their biological activities. Keywords: Silene; Caryophyllaceae; diversity; secondary metabolites; pharmacology; biological properties
2 Diversity 2014, Introduction The genus Silene (family Caryophyllaceae) comprises more than 700 species (allocated in 39 sections) of annuals, biennials, and perennials which are mainly distributed in temperate zones of the Northern emisphere of Eurasia and America, but also in Africa [1,2]. Presently, the genus Silene includes several taxa which were formerly treated as different genera, such as Coronaria, Cucubalus, Lychnis, Melandrium, Petrocopsis, and Viscaria [1]. There are two major centers of diversity in Silene: one in the Mediterranean/Middle East and one in Central Asia. A few taxa have been introduced to other continents. The genus consists mainly of herbaceous plants and, more rarely, small shrubs or subshrubs. The flowers have free petals, with each petal consisting of a usually visible limb that can be divided or entire, and a claw that is included within the synsepalous calyx. Silene has been placed in the tribe Sileneae and the subfamily Caryophylloideae. In molecular phylogenetic studies, the genus Silene clusters in two major clades of approximately equal size, which are tentatively classified as Silene subgenus Silene and Silene subgenus Behen (Moench) Bunge [3,4]. In the most recent taxonomic revision covering the entire genus, Silene has been divided into 44 sections, without any rank above that [5]. Common names of Silene are campion and catchfly. Red Campion (S. dioica), white Campion (S. latifolia, S. alba) and bladder Campion (S. vulgaris) are common wildflowers throughout Europe. Some species of Silene have served as important model plants for studies in ecology, genetics and evolution by famous scientists such as Charles Darwin, Gregor Mendel, Carl Correns, erbert G. Baker, and Janis Antonovics [6]. Silene is an important model system for genetic studies on gynodioecy, dioecy, and polyploidy. Silene also includes a number of cultivated species and widespread weeds [7]. S. acaulis, S. multifida and S. regia have been cultivated as ornamental plants because they produce beautiful flowers [8]. The roots of several species, such as S. latifolia, S. acaulis, S. kumaonensis, and S. conoidea which are rich in saponins with detergent properties, have been traditionally used as a soap substitute for washing clothes similar to other plants of the Caryophyllaceae [9,10]. The soap is obtained by simmering roots in hot water [11,12]. A few species are edible such as S. acaulis, S. cucubalis, and S. vulgaris [13 16]. Especially young shoots and the leaves of S. vulgaris are much appreciated in the traditional gastronomy of Turkey, Italy, Austria, and Spain [14]. A number of Silene species have been used in traditional medicine to treat inflammations, bronchitis, cold, and infections or as a diuretic, antipyretic, analgesic, and emetic [17 24]. Phytoecdysteroids mimic molting hormones of insects and are therefore of interest for chemical ecology and for applications of plant derived insecticides. Because of page restrictions, a thorough review of traditional uses of members of Silene or their pharmacology is out of scope of this review. Silene produces a diversity of secondary metabolites, many of them are important for the plants as defence compounds against herbivores and microbes [25,26]. In this review, the secondary metabolites which have been isolated from the genus Silene are tabulated in detail; the review is based on an analysis of the relevant literature and data bases such as PubMed, Scifinder, and ScienceDirect. The diversity of structures of identified phytochemicals, their names and corresponding plant sources are summarized in Table 1 (below the main text).
3 Diversity 2014, Phytochemical Diversity Phytochemical investigations of the genus Silene have led to the isolation of several phytoecdysteroids [27], triterpene saponins [28], terpenoids, benzenoids, flavonoids [29], anthocyanidins, N-containing compounds [30], sterols, and vitamins [31,32] (see Table 1). The abundance and widespread occurrence of triterpene saponins is a typical feature of the family Caryophyllaceae. f special interest is the presence of phytoecdysteroids which mimic insect molting hormones and which strongly interfere with the metamorphosis of insects. The predominantly edysteroid positive genera of Silene, (including the former genera Coronaria, Lychnis and Petrocoptis) are in the Silenoideae [33 35]. Information on the phytochemistry of the genera Coronaria, Cucubalus, Lychnis, Melandrium, Petrocopsis, and Viscaria is not included, except if it was published under the merged genus Silene. A chemotaxonomical analysis of the data with view on the molecular phylogeny of Silene will be part of a subsequent publication. 3. Biological Properties 3.1. In Vitro Biological Activities Antimicrobial and Antifungal Activities Erturk et al. [8] extracted the apolar fractions from chloroform extract of S. multifida and tested for the antimicrobial activities against six bacteria (Bacillus subtilis, Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, Enterobacter cloacae and Proteus vulgaris and one pathogenic fungus Candida albicans (0.5 mg/ml). All fractions of S. multifida showed activity against all tested bacteria. nly two fractions showed antifungal activity. The oil samples of S. vulgaris and S. cserei subsp. aeoniopsis were also screened against the several standard strains of bacteria and the yeast Candida using the microdilution method [36]. Both of these oils displayed the same activity profile, having notable antibacterial activity against the Gram-negative bacterium Klebsiella pneumoniae at a concentration of 4 g/ml and significant antifungal activity against Candida albicans (16 g/ml). Methanol extracts from three Silene species from Iran (S. gynodioca, S. spergulifolia and S. swertiifolia) were screened for their possible in vitro antibacterial activities by the disc diffusion method [37]. Results indicated that S. swertiifolia has a strong antibacterial activity against three Gram-positive and gram-negative bacteria, namely aemophilus influenzae, Pseudomonas aeruginosa and Bacillus cereus, whereas S. spergulifolia showed a strong inhibition against Bacillus cereus. Bajpai et al. [38] examined the chemical composition of the essential oil isolated from S. armeria and tested the efficacy of essential oil (5 µl/ml, corresponding to 1000 ppm/disc) and different extracts (7.5 µl/ml, corresponding to 1500 ppm/disc) against a diverse range of food spoilage and food-borne pathogens (Bacillus subtilis ATCC6633, Listeria monocytogenes ATCC19166, Staphylococcus aureus KCTC1916, S. aureus ATCC6538, Pseudomonas aeruginosa KCTC2004, Salmonella typhimurium KCTC2515, Salmonella enteritidis KCTC2021, Escherichia coli 157-uman, E. coli ATCC8739, E. coli 57:7 ATCC43888 and Enterobacter aerogenes KCTC2190). The results of this study suggested that the essential oil and leaf extracts derived from S. armeria could be used for the development of novel types of antibacterial agents to control food spoilage and food-borne pathogens.
4 Diversity 2014, We have studied the antimicrobial activity of different extracts and phytoecdysteroids from Silene plants towards pathogenic microorganisms [39]. Acinetobacter spec., Enterococcus faecalis, Klebsiella oxytoca, Pantoea agglomerans, Proteus rettgeri, Pseudomonas aeruginosa and Staphylococcus aureus strains were inhibited by the methanol extract of S. wallichiana (Minimal Inhibitory Concentration, MIC = 2.5 mg/ml), while Escherichia coli and Klebsiella pneumoniae were inhibited with a MIC = 1.25 mg/ml. The butanol extract of S. wallichiana showed activity against pathogenic bacteria Acinetobacter sp., E. coli, K. pneumoniae, P. agglomerans, P. aeruginosa and P. rettgeri, whereas chloroform extract inhibited only Citrobacter freundii, E. coli and P. aeruginosa (MIC = 1.25 mg/ml). The CCl 3 extract of S. brachuica inhibited growth of three Gram-negative (Enterococcus faecalis, Proteus rettgeri, and Pseudomonas aeruginosa) and one Gram-positive (Micrococcus luteus) bacterial strain. The CCl 3 extract of S. viridiflora was active against M. luteus, P. rettgeri, Klebsiella pneumoniae, and P. aeruginosa, whereas the extract of S. wallichiana exhibited activity against only pathogenic bacteria E. coli, M. luteus and P. aeruginosa [40]. Pure phytoecdysteroids (viticosterone E, 20-hydroxyecdysone-22-benzoate, 2-deoxy-20-hydroxyecdysone, 2-deoxyecdysone, 20-hydroxyecdysone and integristerone A) isolated from S. wallichiana exhibited very low activity against the bacteria [39]. Also a preliminary screening of the CCl 3 extract from the aerial part of S. guntensis exhibited antibacterial effects against Escherichia coli, P. aeruginosa, and Acinetobacter sp. [41]. From above-mentioned results we can conclude that the apolar fractions of Silene exhibit moderate activity against both Gram-positive and Gram-negative bacteria, and this activity may be attributed to a synergistic effect, due to the presence of phenols and some monoterpenoids in the apolar fraction Antiviral Activity The lipophilic extracts of a S. vulgaris were tested against the DNA virus erpes simplex (SV) and RNA virus Parainfluenza (PI-3) using Madin-Darby bovine kidney and Vero cell lines [42]. The extracts exerted substantial antiviral effects against both viruses, as compared to acyclovir and oseltamivir. Some plants which have a high amount of palmitic acid were previously reported to have a powerful antiviral activity against. simplex (SV) and Parainfluenza viruses (PIV) [43]. owever, no correlation was found between antiviral activity and fatty acid contents of the extracts Antioxidant Activity Methanol extracts from three Silene species from Iran (S. gynodioca, S. spergulifolia and S. swertiifolia) were screened for their possible in vitro antioxidant activities by three complementary test systems, namely DPP free radical-scavenging, metal chelating activity and β-carotene/linoleic acid oxidation [37]. Results showed that S. swertiifolia, which contains high amount of phenolics and flavonoids, exhibited the greatest antioxidant activity. The extracts of S. swertiifolia and S. spergulifolia showed a higher potency than ascorbic acid in scavenging of DPP free radical. In the metal-chelating assay all extracts had a lower activity than ascorbic acid. In the β-carotene/linoleic acid system, oxidation of linoleic acid was effectively inhibited by the S. swertiifolia extract. The radical scavenging activity of the plant extracts decreased in the following order: ascorbic acid (IC 50 = 0.13 mg/ml) > S. swertiifolia (IC 50 = 0.13 mg/ml) > S. spergulifolia (IC 50 = 0.21 mg/ml) > S. gynodioca (IC 50 = 0.29 mg/ml).
5 Diversity 2014, Conforti et al. [44] studied the in vitro antioxidant activity of the hydroalcoholic extract from S. vulgaris. A very good correlation between radical scavenging activity and polyphenol content (for S. vulgaris 67.5 mg/g of extract) was found. Taskin and Bitis [45] reported that S. alba subsp. divaricata leaves have beneficial effects on ferrous chelating, DPP radical-scavenging and ABTS radical cation scavenging abilities. This plant contained the highest phenolic compounds and may thus exert protection against oxidative damage. The radical scavenging ability of the extracts and phytoecdysteroids of S. guntensis were evaluated by us using the reaction with the stable DPP radical [46]. In our experiments phytoecdysteroids were ineffective for DPP radical scavenging activity (IC 50 value > 100 µg/ml). Maximum scavenging activity of DPP was observed with the water extract (IC μg/ml) of S. guntensis, followed by the activities of the butanol, methanol, and chloroform extracts with IC 50 values of 69.12, , and μg/ml, respectively. The activities of 20-hydroxyecdysone, 2-deoxy-20-hydroxyecdysone, and 2,3-diacetate-22-benzoate-20-hydroxyecdysone were , , and μg/ml, respectively. owever, we assume that the antioxidant effect of these extracts might be attributed to some co-eluting phenolic compounds and not to phytoecdysteroids, lipids etc Phagocytic Activity Popov et al. [47] studied the effects of the polysaccharides from plants and callus of S. vulgaris (silenans) on uptake capacity and myeloperoxidase activity in the peripheral human neutrophils and monocytes and rat peritoneal macrophages in vitro. All polysaccharides (three silenans from the intact plant; pectic polysaccharides P1, P2 and P3) and two from the callus (acidic arabinogalactan C1 and pectin C2) enhanced uptake capacity at concentration of 15 mg/ml. The acidic arabinogalactan C1 was only found to stimulate lysosomal activity of the peripheral phagocytes. The effect of some polysaccharides was established in peritoneal resident macrophages. Pectins P1, P3 and C2 failed to enhance myeloperoxidase activity of the macrophages in calcium-free solution, whereas arabinogalactan C1 was independent of extracellular calcium. Polysaccharides studied failed to influence either complement receptor CR3- or scavenger receptor SR-mediated adhesion of the macrophages. The data obtained demonstrate that the S. vulgaris may be used as sources of immunoactive polysaccharides and that pectins and weakly acidic arabinogalactan seem to stimulate macrophages through different mechanisms. Complement receptor type 3 and scavenger receptor failed to mediate the cell activation induced by plant polysaccharides Inhibition of Nitric xide (N) Production Conforti et al. [44] examined whether S. vulgaris can modulate the production of N by the RAW mouse macrophage cell line pre-treated with a hydroalcoholic extract ( μg/ml) prior to activation by bacterial lipopolysaccharide (LPS). The treatment of RAW macrophages with LPS (1 μg/ml) for 24 h, induced N production which can be quantified by utilising the chromogenic Griess reaction and measuring the accumulation of nitrite, a stable metabolite of N. The beneficial effect of extracts on the quenching of inflammatory mediators in macrophages can be mediated through oxidative degradation of phagocytosis products, such as 2 and Cl. S. vulgaris had a weak cytotoxicity (202 ± 2.6 μg/ml), while the reference drug indomethacin showed cytotoxicity with IC 50 = 58 μg/ml.
6 Diversity 2014, Antitumor Activity In our in vitro experiments pure compounds, such as phytoecdysteroid 2,3-diacetate-22-benzoate- 20-hydroxyecdysone showed a moderate inhibition against ela and epg-2 cells (IC 50 values ( ± μm) and ( ± 7.81 μm), respectively), while 2-deoxy-20-hydroxyecdysone inhibited MCF-7 cells at a concentration IC 50 = ± μm [46]. Conforti et al. [44] reported that a hydroalcoholic extract from S. vulgaris showed a weak cytotoxicity against the murine monocytic macrophage cell line RAW (IC 50 = 712 μg/ml). Behzad et al. [48] also informed that S. ampulata, S. peduncularis plants showed no cytotoxic activity (IC 50 > 100 μl/ml) against normal and cancer cell lines. The triterpene saponins from the roots of S. fortunei were tested in an in vitro lymphocyte proliferation assay. The saponins, jenisseensosides C and D and their deacylated derivatives stimulated the proliferation of the Jurkat tumor cell lines (human T-cell leukaemia) at low concentrations (1 nm to 5 μm). At high concentrations (>10 μm), they inhibited the proliferation of the cells probably due to the induction of apoptosis [28]. These authors [49] reported that the trans- and cis-p-methoxycinnamoyl triterpene saponins jenisseensosides A to D (from S. jenisseensis and S. fortunei) increased the accumulation and cytotoxicity of the anticancer agent cisplatin in T 29 (human colon tumor) cells In Vivo Biological Activities Antitumor Activity Zibareva [50] reported that the ecdysteroid-containing extract of S. viridiflora exerted antitumor activity in vivo, however investigations with individual phytoecdysteroids showed no effect [51]. Also, El-Mofti [52,53] reported that ecdysone was able to induce neoplastic lesions in toads and mice, a result which appears somewhat surprising when considering the very low doses of ecdysone used. The phytoecdysteroids cyasterone, polypodine B, and decumbesterone A showed potent antitumor activities in a mouse-skin model in vivo in a two-stage carcinogenesis trial, using 7,12-dimethylbenz[a]anthracene as initiator and 12--tetradecanoylphorbol-13-acetate (TPA) as promoter [54]. owever, Lagova and Valueva [55] reported that 20-hydroxyecdysone was mainly ineffective in preventing tumor growth in mice, but it stimulated the growth of mammary gland carcinomas. Because ecdysteroids structurally resemble sex hormones, they might indeed bind to steroid hormone receptors in mammals and stimulate the growth of hormone-dependent tumors. Binding studies performed so far for 20-hydroxyecdysone and a set of phytoecdysteroids [56,57] do not support this hypothesis, but they were not performed with all in vivo metabolites Immunomodulatory Activity The total ecdysteroid preparation from S. viridiflora for immunostimulation in vivo was analyzed by Shakhmurova et al. [58]. The preparation (5 mg/kg) acts as an effective immunomodulator in normal mice and in mice with secondary immunodeficiency developed under irradiation, and with acute toxic hepatitis. The immunomodulating activity of total ecdysteroids from S. viridiflora is comparable with that of the known immunity stimulator T-activin, a polypeptide preparation from cattle thymus. Furthermore, Bushneva et al. [59] showed that pectic polysaccharide named silenan which was
7 Diversity 2014, isolated from the aerial parts of S. vulgaris, possess immunomodulatory activity. Ghonime et al. [60] confirmed the immunomodulatory activity Silene species. Extracts from S. nocturna were examined for their immunomodulatory effect in Balb/c mice. Treatment (intraperitoneal injection) with five doses of the methanol extract enhanced the total white blood cells count (up to cells/mm 3 ). Bone marrow cell density also increased significantly after the administration of the extract. Furthermore, spleen weight of the treated groups was significantly increased as compared to controls. Two groups of mice were immunosuppressed with cyclophosphamide; the one which was pre-treated with S. nocturna extracts significantly restored their resistance against lethal infection with the predominantly granulocyte-dependant Candida albicans Adaptogen and Actoprotection Activity The total ecdysteroid preparation from S. viridiflora ( Siverinol ) and S. brachuica ( Silekbin ) was analyzed for actoprotector and adaptogenic activity in vivo by several researchers [61 64]. Siverinol (oral intake doses ranged between 100 and 3000 mg/kg b.w. over 14 days) increased endurance (swimming tests) and reduced the recovery time (lactic acid recycling, regeneration of glycogen stores) after a severe physical load. Chronic exposure over 7 14 days resulted in a significant stimulation of erythropoiesis and increase of muscle size. Moreover, it reduced the stress effects of an extended physical exercise. The pharmacocorrective influence of Siverinol and Silekbin to biochemical mechanisms of dis-adaptation and basal processes of bioenergetics in the muscle tissue of the experimental animals was the base of actoprotector activity epatoprotection Activity The effect of an oral administration of a 50% ethanol extract from S. aprica on acute liver injury was examined in rats intoxicated with carbon tetrachloride and acetaminophen [65]. The results indicated that S. aprica protected the liver intoxication as judged by morphological and biochemical observations. An increase in both lipid peroxidation and triglyceride concentrations occurred in the liver after carbon tetrachloride injection; S. aprica administration significantly reduced these changes. Also Shin et al. [66] reported that a S. takesimensis extract or a mixed extract with Melandrium firmum relieved fibrotic liver damage induced by carbon tetrachloride through inhibition of ALT and AST enzymes in the liver. The extracts inhibited hepatic fibrosis without affecting liver stromal cells by decreasing the amount of collagen, alpha-smooth muscle actin, and TGF-β inside the liver tissues Electrical Activity of the eart Golovko and Bushneva [21] studied the effect of silenan (a pectin polysaccharide from S. vulgaris), during development of arrhythmia and in disorders of cell-cell interactions in the zone of contact between the venous sinus and atrial cells. Electrical activity of myocardial cells was studied with spontaneously contracting strips from the sinoatrial area of Rana temporaria heart. Silenan corrected disorders in the conduction of action potentials between cells of the sinoatrial area of frog heart forming a functional syncytium. Recovery of action potential conduction in the sinoatrial cells was recorded in long-term experiments (>8 h). The effect of silenan mainly concerned the background of arrhythmic generation and impaired propagation of action potentials.
8 Diversity 2014, Insecticidal Activity Phytoecdysteroids are analogues of insect molting hormones and sometimes their concentration in plants can reach 0.01% 3%. Even at ultralow concentrations they can affect insect development. For example, ecdysteroid 20-hydroxyecdysone at concentrations of 10 8 to 10 9 M initiates the transformations occurring in embryogenesis and during larval development with instant metamorphosis to the adult insect [67]. The potential insect deterrent activity of several Silene species, such as S. conoidea, S. ampulata and S. peduncularis, have been reported by several authors [48,68]. Chermenskaya et al. [69] reported that ethanol extracts of the aerial parts of S. sussamyrica showed substantial insecticidal activity, especially against western flower thrips larvae Frankliniella occidentalis Perg. (Thysanoptera: Thripidae). In this case we can assume this plant probably contained phytoecdysteroids and these compounds caused death of F. occidentalis larvae. 4. Conclusions The genus Silene is known to be a source of biological active compounds. Phytochemical analysis of Silene demonstrated their richness in various compounds (>450 compounds have been isolated) belonging to different structural types, such as phytoecdysteroids, triterpene saponins, terpenoids, benzenoids, flavonoids, N-containing compounds, sterols, vitamins and others. The most prominent compounds in Silene species are the phytoecdysteroids, which have a similar chemical structure to molting hormones of insects. From data collected in this review, it is evident that the genus Silene comprises a wide range of pharmaceutically promising, interesting, and valuable plants. Some species of the genus Silene are used as ornamental plants and in folk medicine to treat inflammations, bronchitis, cold, and infections or as a diuretic, antipyretic, analgesic, emetic, etc. Many of the traditional uses have been validated by scientific research. It would be important for future studies to include the former genera Coronaria, Cucubalus, Lychnis, Melandrium, Petrocopsis, and Viscaria, which presently are partly included in the larger genus Silene [1]. The collected data provides a means to understand the latest developments in the pharmacology and phytochemistry of the genus Silene. Current pharmacological data is in many cases limited to studies on plant extracts and, hence, efforts are needed towards the isolation of biologically active compounds. Due to their various promising activities, further studies are warranted to be carried out on the drug development of Silene extracts and their constituents. Acknowledgements We would particularly like to express our gratitude to Mohamed L. Ashour for his help in collecting references for this paper. Author Contributions Nilufar Mamadalieva collected the information and drafted the manuscript which was interpreted and edited by Michael Wink. Rene Lafont conducted the biological part of the manuscript. All authors read and finally approved the final review.
9 Diversity 2014, Table 1. Structures and Distribution of Secondary Metabolites in the Genus Silene. Triterpenoids R2 R3 Phytoecdysteroids R R6 R R8 25 R7 27 R4 Structure Name Substituents in Steroidal Core Substituents in Side-Chain Plant Source Reference R 1 R 2 R 3 R 4 R 5 R 6 R 7 R 8 Brahuisterone C 3 S. brahuica Boiss [70] 2-Deoxy-20,26- dihydroxyecdysone 22-Deoxy-20,26- dihydroxyecdysone C 2 S. pseudotites Bess ex Reichenb [71,72] C 2 S. nutans L. [73,74] 2-Deoxyecdysone C 3 S. brahuica Boiss, S. claviformis Litv, S. fridvaldszkyana ampe, S. gigantea L., S. graminifolia tth, S. latifolia (Gilib) Aschers, S. otites (L.) Wibel, S. praemixta M Pop, S. pseudotites Bess ex Reichenb, S. repens Patrin, S. roemeri Friv, S. scabrifolia Kom, S. tomentella Schischk, S. wallichiana Klotsch [71,72,75 98] 2-Deoxyecdysone-3-acetate Ac C 3 S. scabrifolia Kom [99]
10 Diversity 2014, Structure Name Substituents in Steroidal Core Substituents in Side-Chain Plant Source Reference R 1 R 2 R 3 R 4 R 5 R 6 R 7 R 8 2-Deoxyecdysone-22- acetate 2-Deoxyecdysone-22- benzoate 2-Deoxyecdysone-22- glucoside 2-Deoxy-20- hydroxyecdysone 2-Deoxy-20- hydroxyecdysone-3-acetate 5α-2-Deoxy-20- hydroxyecdysone-3-acetate 2-Deoxy-20- hydroxyecdysone-22- acetate 2-Deoxy-20- hydroxyecdysone-25- acetate Ac C 3 S. brahuica Boiss, S. otites (L.) Wibel [74,100] Bz C 3 S. wallichiana Klotsch [85] Glu C 3 S. praemixta M Pop, S. pseudotites Bess ex Reichenb [71,72,98] S. antirrhina L., S. brahuica Boiss, S. chlorifolia Smith, S. claviformis Litv, S. cretica L., S. disticha Willd, S. fridvaldszkyana ampe, S. gigantea L., S. guntensis B Fredtsch, S. italica (L.) Pers, S. italica ssp. C 3 nemoralis, S. latifolia (Gilib) Aschers, S. linicola [46,71,72,76 79,81,82,85 94,96 98, ] C.C.Gmelin., S. otites (L.) Wibel, S. portensis L., S. praemixta M Pop, S. pseudotites Bess ex Reichenb, S. repens Patrin, S. roemeri Friv, S. scabrifolia Kom, S. viridiflora L., S. wallichiana Klotsch Ac C 3 S. otites (L.) Wibel, S. praemixta M Pop [114,115] Ac (α) C 3 S. otites (L.) Wibel [114] Ac C 3 S. otites (L.) Wibel [74] Ac C 3 S. wallichiana Klotsch [116]
11 Diversity 2014, Structure Name Substituents in Steroidal Core Substituents in Side-Chain Plant Source Reference R 1 R 2 R 3 R 4 R 5 R 6 R 7 R 8 2-Deoxy-20- hydroxyecdysone-3- Bz C 3 S. wallichiana Klotsch [77] benzoate 2-Deoxy-20- S. nutans L., S. otites (L.) Wibel, S. supina Bieb, hydroxyecdysone-22- Bz C 3 [74,81,91,101, ] S. tatarica (L.) Wild benzoate 2-Deoxy-20- hydroxyecdysone-3- CC 2 2 C 3 C 3 S. otites (L.) Wibel [114] crotonate 2-Deoxy-20- hydroxyecdysone-3,22- Ac Ac C 3 S. otites (L.) Wibel [114] diacetate 2-Deoxy-20- hydroxyecdysone-22- -β-d-glu C 3 S. italica ssp. nemoralis [104] glucoside 2-Deoxy-20- hydroxyecdysone-25- -β-d-glu C 3 S. gigantea L. [91,92,95] glucoside 2-Deoxyintegristerone A C 3 S. italica ssp. nemoralis, S. otites (L.) Wibel, S. pseudotites Bess ex Reichenb, S. viridiflora L. [74,102,105,112,122] 5α-2-Deoxyintegristerone S. italica ssp. nemoralis, S. pseudotites Bess ex C 3 A (α) Reichenb [72,110] 22-Deoxyintegristerone A C 3 S. italica ssp nemoralis, S. nutans L. [74,105] 5α-22-Deoxyintegristerone A (α) C 3 S. nutans L. [74]
12 Diversity 2014, Structure Name Substituents in Steroidal Core Substituents in Side-Chain Plant Source Reference R 1 R 2 R 3 R 4 R 5 R 6 R 7 R 8 2-Deoxypolypodine B-3-glucoside -β-d-glu C 3 S. pseudotites Bess ex Reichenb, S. viridiflora L. [71,72,123] 2-Deoxy-5,20,26- trihydroxyecdysone C 2 S. viridiflora L. [122] 20,26-Dihydroxyecdysone S. fridvaldszkyana ampe, S. nutans L., S. otites (L.) C 2 (Podecdysone C) Wibel., S. viridiflora L. [71,74,76,88 90,93 95,124] 20,26-Dihydroxyecdysone- 2,22-diacetate Ac Ac C 2 S. viridiflora L. [71,125] 20,26-Dihydroxyecdysone- 3,22-diacetate Ac Ac C 2 S. viridiflora L. [71,125] Ecdysone C 3 S. cretica L., S. disticha Willd, S. echinata tth, S. italica (L.) Pers., S. italica ssp. nemoralis, S. linicola C.C.Gmelin., S. otites (L.) Wibel, S. portensis L., [71,72,88 90,92,93,96,107,109, ] S. praemixta M Pop, S. pseudotites Bess. ex Reichenb, S. radicosa Bois et eldr Ecdysone-22-sulfate S 3 C 3 S. brahuica Boiss [126] Ecdysteroside -α-d-gal (1 6) C 3 S. tatarica (L.) Wild [127] α-d-gal 5α-20-ydroxyecdysone (α) C 3 S. italica ssp. nemoralis [110] 5α-20-ydroxyecdysone- 22-benzoate (α) Bz C 3 S. scabrifolia Kom [128]
13 Diversity 2014, Structure Name Substituents in Steroidal Core Substituents in Side-Chain Plant Source Reference R 1 R 2 R 3 R 4 R 5 R 6 R 7 R 8 20-ydroxyecdysone C 3 S. acaulis (L.) Jacg, S. altaica Pers, S. ambigua Turcz, S. antirrhina L., S. apetala Willd, S. aprica Turel, S. armeria L., S. bashkirorum Janish, S. bellidifolia Juss. ex Jacq, S. bergiana Lindm, S. borystenica (Gruner) Walters, S. bourgeaui. Christ, S. brachypoda Rouy, S. brahuica Boiss, S. burchelli tth, S. campanulata, S. Watson, S. caramanica Boiss, S. catholica (L.) Aiton fil, S. caucasica Boiss, S. chamarensis Turcz, S. chlorantha Willd, S. chlorifolia Smith, S. ciliata Pourret, S. ciliata var graefteri (P), S. claviformis Litv, S. coeli-rosa (L.) Godron in Gren, S. colorata Poiret, S. colorata ssp. trichocalysina, S. coronaria (L.) Clairv, S. cretaceae Fisch, S. cretica L., S. damboldtiana Greuter et Melzh, S. densiflora (L.) Wib. Drurv, S. dioica (L.) Clairv, S. disticha Willd, S. echinata tth, S. elegans L., S. fetissovii Lazkov, S. firma Siebold et Zucc, S. flavescens Waldst et Kit, S. foliosa Maxim, S. fridvaldszkyana ampe, S. fruticosa L., S. fruticulosa (Pall.) Schishk, S. gallica L., S. gallica var. quiquivulnera (L.) Koch, S. gebleriana Schrenk, S. gigantea L., S. goulimyi Turrill, S. graefferi Guss, S. graminifolia tth, S. guntensis B Fredtsch, S. hifacensis Rouy ex willk, S. holopetala Lebed, S. ichebogda Glub, S. incurvifolia Kar et Kir, S. italica (L.) Pers, S. italica [46,71,72,75,78 93,95 98,101,104, , ,115,117,121, ]
14 Diversity 2014, Structure Name Substituents in Steroidal Core Substituents in Side-Chain Plant Source Reference R 1 R 2 R 3 R 4 R 5 R 6 R 7 R 8 20-ydroxyecdysone C 3 ssp. nemoralis, S. jenisseensis Willd, S. kungessana B Fedtsch, S. latifolia (Gilib) Aschers, S. linicola C.C.Gmelin, S. longicalycina Kom, S. longicilia (Brot) tth, S. mellifera Boiss. et Reuter, S. melzheimeri Greuter, S. micropetala Lag, S. mollissima (L.) Pers, S. mongolica Maxim, S. multicaulis Guss, S. multiflora (Waldst et Kit) Pers, S. nemoralis Waldst et Kit, S. nutans L., S. obovata Schischk., S. odoratissima Bunge, S. oligantha Boiss, S. otites (L.) Wibel, S. otites var. parviflorus, S. paradoxa L., S. parnassica Boiss, S. patula Desf, S. portensis L., S. praemixta M Pop, S. pseudotites Bess. ex Reichenb, S. psevdovelutina Rothm, S. pygmaea Adams, S. quinquevulnera L., S. radicosa Bois et eldr, S. regia, S. reichenbachii Vis, S. repens Patrin, S. roemeri Friv, S. rubella L., S. saxatilis Sims, S. saxifraga L., S. scabriflora Brot, S. scabrifolia Kom, S. schafta S.G.Gmel. ex ohen, S. schischkinii (M Pop) Vved, S. schmuckeri Wettst, S. secundiflora tth, S. sendtneri Boiss, S. sericea All, S. sieberi Fenzl, S. sobolevskajae Czer, S. supina Bieb, S. spergulifolia (Willd) Bieb, S. squamigera Boiss, S. stenophylla Ledeb, S. stylosa Bunge, S. sussamyrica Lazkov, S. tatarica (L.) Wild, S. thessalonica Boiss et eldr, S. tomentella Schischk, S. turchaninova Lazkov, S. turgida L., S. uralensis (Rupr) Bocquet, S. viridiflora L., S. viscosa (L.) Pers, S. wallichiana Klotsch, S. wolgensis (ornem) tth, S. zawadskii erbich [46,71,72,75,78 93,95 98,101,104, , ,115,117,121, ]
15 Diversity 2014, Structure Name Substituents in Steroidal Core Substituents in Side-Chain Plant Source Reference R 1 R 2 R 3 R 4 R 5 R 6 R 7 R 8 20-ydroxyecdysone-2- acetate Ac C 3 S. otites (L.) Wibel [102,112] 20-ydroxyecdysone-3- acetate Ac C 3 S. otites (L.) Wibel [102,112] 20-ydroxyecdysone-22- acetate Ac C 3 S. otites (L.) Wibel [74] 20-ydroxyecdysone-20- benzoate Bz C 3 S. tatarica (L.) Wild [141] 20-ydroxyecdysone-22- S. otites (L.) Wibel, S. scabrifolia Kom, Bz C 3 benzoate S. wallichiana Klotsch [74,83,142] 20-ydroxyecdysone-22- benzoate-25-glucoside Bz -β-d-glu C 3 S. otites (L.) Wibel [74] 20-ydroxyecdysone -2,3- diacetate-22-benzoate Ac Ac Bz C 3 S. guntensis B Fredtsch [46] 20-ydroxyecdysone- 22,25-dibenzoate Bz Bz C 3 S. scabrifolia Kom [142] 20-ydroxyecdysone-3- glucoside -β-d-glu C 3 S. otites (L.) Wibel [103] 20-ydroxyecdysone-25- glucoside -β-d-glu C 3 S. otites (L.) Wibel [74] 26-ydroxyintegristerone A C 2 S. fridvaldszkyana ampe [95] 26-ydroxypolypodine B C 2 S. fridvaldszkyana ampe, S. nutans L., S. viridiflora L. [71,74,95,108] Inokosterone C 2 S. disticha Willd, S. pseudotites Bess. ex Reichenb, S. regia Sims [72,95,112]
16 Diversity 2014, Structure Name Substituents in Steroidal Core Substituents in Side-Chain Plant Source Reference R 1 R 2 R 3 R 4 R 5 R 6 R 7 R 8 Integristerone A C 3 S. brahuica Boiss., S. claviformis Litv, S. fridvaldszkyana ampe, S. gigantea L., S. italica ssp. nemoralis, S. nutans L., S. otites (L.) Wibel, S. repens Patrin, S. scabrifolia Kom, S. supina Bieb, S. tatarica (L.) [74,77 81,83,86,87,92,95,104,108,112,135,143] Wild, S. tomentella Schischk, S. viridiflora L., S. wallichiana Klotsch Integristerone A-25-acetate Ac C 3 S. brahuica Boiss [144] S. altaica Pers, S. antirrhina L., S. brachypoda Rouy, S. brahuica Boiss, S. campanulata S. Watson, S. caramanica Boiss, S. catholica (L.) Aiton fil, S. caucasica Boiss, S. chlorifolia Smith, S. ciliata Pourret, S. cretica L., S. damboldtiana Greuter et Melzh, S. disticha Willd, S. echinata tth, S. fridvaldszkyana Polypodine B C 3 S. nutans L., S. paradoxa L., S. parnassica Boiss, S. pseudotites Bess. ex Reichenb, S. radicosa Bois et eldr, S. regia Sims, S. repens Patrin, S. roemeri Friv, S. schmuckeri Wettst, S. sendtneri Boiss, S. supina Bieb, S. tatarica (L.) Wild, S. tomentella Schischk, S. viridiflora L. ampe, S. italica (L.) Pers, S. italica ssp. nemoralis, [71,72,78 81,84,86,88 93,95 97,101,104, S. linicola C.C.Gmelin, S. mellifera Boiss et Reuter, , ,118,131,135] S. antirrhina L., S. brahuica Boiss, S. chlorifolia Smith, S. disticha Willd, S. echinata tth, S. italica Ponasterone A C 3 (L.) Pers, S. portensis L., S. pseudotites Bess. ex Reichenb, S. radicosa Bois et eldr, S. regia Sims [50,71,72,88 90,92,94,96,106, ]
17 Diversity 2014, Structure Name Substituents in Steroidal Core Substituents in Side-Chain Plant Source Reference R 1 R 2 R 3 R 4 R 5 R 6 R 7 R 8 Sileneoside A -α-d-gal C 3 S. brahuica Boiss, S. nutans L., S. scabrifolia Kom, S. supina Bieb, S. tatarica (L.) Wild, S. viridiflora L. [95,108,112,135] Sileneoside B -β-d-gal -β-d-gal C 3 S. brahuica Boiss [136] Sileneoside C -α-d-gal C 3 S. brahuica Boiss [137] Sileneoside D -β-d-gal C 3 S. brahuica Boiss, S. scabrifolia Kom, S. supina Bieb, S. tatarica (L.) Wild, S. viridiflora L. [108,112,143,145] Silenoside E (Blechnoside A) -β-d-glu C 3 S. brahuica Boiss [84] 5α-Silenoside E -β-d-glu (α) C 3 S. brahuica Boiss [146] Sileneoside F -β-d-glu C 3 S. brahuica Boiss [147] Sileneoside G -α-d-glu -α-d-gal C 3 S. brahuica Boiss [148] Sileneoside -α-d-gal Ac C 3 S. brahuica Boiss [149] Taxisterone C 3 S. italica ssp. nemoralis, S. nutans L., S. viridiflora L. [104,150,151] Tomentesterone A (α) Ac Bz C 3 S. tomentella Schischk [80] Tomentesterone B (α) Bz C 3 S. tomentella Schischk [152] Viticosterone E Ac C 3 S. brahuica Boiss, S. linicola C.C.Gmelin, S. otites (L.) Wibel, S. praemixta M Pop, S. tomentella Schischk, [80,85,102,107,112,115,135] S. wallichiana Klotsch Viticosterone E-22-benzoate Ac Ac C 3 S. wallichiana Klotsch [104,153]
18 Diversity 2014, R6 R7 R8 R5 R1 R2 R3 R4 Name R 1 R 2 R 3 R 4 R 5 R 6 R 7 R 8 Plant Source Reference 24(28)-Dehydromakisterone A C 3 C 2 S. fridvaldszkyana ampe, S. italica ssp. nemoralis, S. otites (L.) Wibel, S. roemeri Friv [71,76,88 90,92 95,104,112] 2-Deoxy-21-hydroxyecdysone C 2 S. otites (L.) Wibel, S. pseudotites Bess. ex Reichenb [102,112] 5α-2-Deoxy-21-hydroxyecdysone (α) C 2 S. otites (L.) Wibel [102] 9α,20-Dihydroxyecdysone (α) C 3 S. italica ssp. nemoralis [109,110] 9β,20-Dihydroxyecdysone C 3 S. italica ssp. nemoralis [110] Makisterone A C 3 C 3 S. otites (L.) Wibel [112] Nusilsterone C 3 S. nutans L. [154] Turkesterone C 3 S. linicola C. C. Gmelin [107] R4 R3 R1 R2 Name R 1 R 2 R 3 R 4 Plant Source Reference 2-Deoxy-20-hydroxyecdysone-20,22-acetonide C 3 S. viridiflora L. [122] 5α-2-Deoxy-20-hydroxyecdysone-20,22-acetonide (α) C 3 S. viridiflora L. [155] 2-Deoxy-5,20,26-trihydroxyecdysone-20,22-acetonide C 2 S. viridiflora L. [122] 20,26-Dihydroxyecdysone-20,22-acetonide C 2 S. viridiflora L. [122]
19 Diversity 2014, ydroxyecdysone-20,22-acetonide C 3 S. scabrifolia Kom [156] 20-ydroxyecdysone 20,22-acetonide-25-acetate Ac C 3 S. viridiflora L. [123] 5,20,26-Trihydroxyecdysone-20,22-acetonide C 2 S. viridiflora L. [122] 20-ydroxyecdysone-2,3-acetonide R 1 = R1 S. scabrifolia Kom [142] 20-ydroxyecdysone-2,3-acetonide-22- R 1 = Bz benzoate S. scabrifolia Kom [83,142] 5α-Dihydro rubrosterone R 1 = (α) S. otites (L.) Wibel [103] 5β-Dihydro rubrosterone R 1 = S. otites (L.) Wibel [103] 20, 22-Acetal isovaleric aldehyde-5β- cholest-7-en-2β,3β,14α,20r,22r,25- hexahydroxy-6-on 20,22-Acetal epiisovaleric aldehyde-5β- cholest-7-en-2β,3β,14α,20r,22r,25- hexahydroxy-6-on R1 R1 R 1 = (α) S. claviformis Litv [87] R 1 = S. claviformis Litv [87]
20 Diversity 2014, Dihydropoststerone S. otites (L.) Wibel [74] Poststerone S. otites (L.) Wibel [74] Poststerone S. otites (L.) Wibel [74] Rubrosterone S. otites (L.) Wibel [74]
21 Diversity 2014, Makisterone C-2,3;20,22-diacetonide S. viridiflora L. [155] Praemixisterone S. praemixta M Pop [134] Sidisterone S. dioica (L.) Clairv, S. otites (L.) Wibel, S. pseudotites Bess. ex Reichenb [86,90,92,94,106,112,133] Silenosterone S. praemixta M Pop [82]
22 Diversity 2014, Triterpene Saponins CR2 R C 27 β-d-galactopyranosyl-(1 2)-β-D-glucuronopyranosyl-3β-hydroxy-23-oxoolean- 12-en-28-oic acid 28--β-D-xylopyranosyl(1 3)-β-D-xylopyranosyl(1 4)-α-Lrhamnopyranosyl (1 2)-β-D-fucopyranoside (Silenosides A) R 1 = -β-d-glcuap-2 1-β-D-Galp R 2 = -β-d-fucp-2 1-α-L-Rhap-4 1-β-D-Xylp-3 1-β-D-Xylp S. vulgaris (Moench) Garcke (syn. S. inflata) [157] 2 1-β-D-Galp 3--{β-D-Galactopyranosyl-(1 2)-[β-D-xylopyranosyl]-(1 3)]-β-D- R 1 = -β-d-glcuap- R 2 = -β-d-fucp- 3 1-β-D-Xylp glucuronopyranosyl}-28--{β-d-xylopyranosyl-(1 3)-β-D-xylopyranosyl-(1 4)- α-l-rhamnopyranosyl-(1 2)]-[3,4-di--acetyl-β-D-quinovopyranosyl-(1 4)]-β- D-fucopyranosyl] gypsogenin (Silenorubicunoside A) 2 1-α-L-Rhap-4 1-β-D-Xylp- 3 1-β-D-Xylp S. rubicunda Franch [158] 4 1-(3,4-di--Ac)-β-D-Quip 3--{β-D-Galactopyranosyl-(1 2)-[β-D-xylopyranosyl]-(1 3)]-β-Dglucuronopyranosyl}-28--{β-D-xylopyranosyl-(1 4)-α-L-rhamnopyranosyl- (1 2)-3,4-di--acetyl-β-D- fucopyranosyl] gypsogenin (Silenorubicunoside C) 2 1-β-D-Galp R 1 = -β-d-glcuap- 3 1-β-D-Xylp R 2 = -(3,4-di--Ac)-β-D-Fucp-2 1-α-L-Rhap-4 1-β-D-Xylp S. rubicunda Franch [158]
23 Diversity 2014, {β-D-Galactopyranosyl-(1 2)-[β-D-xylopyranosyl-(1 3)]-β-D- glucuronopyrannosyl}-28--{β-d-xylopyranosyl-(1 3)-β-D-xylopyranosyl- (1 4)- α-l-rhamnopyranosyl-(l 2)-[3,4-di--acetyl-β-D-fucopyranosyl} gypsogenin (Glanduloside C) Gypsogenin 3--β-xylopyranosyl-(1 3)-[β-galactopyranosyl-(1 2)]-βglucuronopyranside Nutanoside Gypsogenin 3--glucuronide Gypsogenin 3--glycoside 2 1-β-D-Galp R 1 = -β-d-glcuap- 3 1-β-D-Xylp R 2 = -(3,4-di--Ac)-β-D-Fucp-2 1-α-L-Rhap-4 1-β-D-Xylp-3 1-β-D-Xylp 2 1-β-D-Galp R 1 = -β-d-glcuap- 3 1-β-D-Xylp R 2 = 6 1-α-L-Arap R 1 = -β-d-glcuap-3 1-β-D-Galp- 4 1-β-D-Xylp 3 1-β-D-Galp-2 1-β-D-Fucp- 4 1-β-D-Glcp R 2 = -α-l-rhap- 4 1-β-D-Glcp R 1 = -β-d-glcuap, R 2 = R 1 = -β-d-glcp, R 2 = S. rubicunda Franch [158] S. cucubalus Wib [159] S. nutans L. [160] S. vulgaris (Moench) Garcke [161] S. vulgaris (Moench) Garcke [161]
24 Diversity 2014, R C CR2 3--[β-D-Galactopyranosyl-(1 2)-β-D-glucuronopyranosyl]- 28--[β-D- R 1 = -β-d-glcuap-2 1-β-D-Galp glucopyranosyl-(1 2)-α-L-rhamnopyranosyl-(1 2)-β-D-4--trans-p- S. jenisseensis [162,1 methoxycinnamoyl- fucopyranosyl] quillaic acid (Jenisseensoside A) R 2 = (4--E-p-methoxycinnamoyl-)-β-D-Fucp-2 1-α-L-Rhap- 2 1-β-D-Glcp Willd 63] 3--[β-D-Galactopyranosyl-(1 2)-β-D-glucuronopyranosyl]- 28--[β-D- R 1 = -β-d-glcuap-2 1-β-D-Galp glucopyranosyl-(1 2)-α-L-rhamnopyranosyl-(1 2)-β-D-4--cis-p- S. jenisseensis [162,1 methoxycinnamoyl- fucopyranosyl] quillaic acid (Jenisseensoside B) R 2 = (4--Z-p-methoxycinnamoyl-)-β-D-Fucp-2 1-α-L-Rhap- 2 1-β-D-Glcp Willd 63] 3--β-D-Galactopyranosyl-(1 2)-β-D-glucuronopyranosyl- 28--[{α-L- R 1 = -β-d-glcuap-2 1-β-D-Galp rhamnopyranosyl -(1 2)}-{4--trans-p-methoxycinnamoyl}-β-D-fucopyranosyl] S. fortunei Wis, S. [163, quillaic acid jenisseensis Willd 164] (Jenisseensoside C) R 2 = (4--E-p-methoxycinnamoyl-)-β-D-Fucp-2 1-α-L-Rhap 3--β-D-Galactopyranosyl-(1 2)-β-D-glucuronopyranosyl- 28--[{α-L- R 1 = -β-d-glcuap-2 1-β-D-Galp rhamnopyranosyl -(1 2)}-{4--cis-p-methoxycinnamoyl}-β-D-fucopyranosyl] S. fortunei Wis, S. [163, quillaic acid jenisseensis Willd 164] (Jenisseensoside D) R 2 = (4--Z-p-methoxycinnamoyl-)-β-D-Fucp-2 1-α-L-Rhap
25 Diversity 2014, [β-D-Galactopyranosyl-(1 2)-β-D-glucuronopyranosyl]quillaic acid-28--α- L-rhamnopyranosyl-(1 2)-3--acetyl-4--trans-p-methoxycinnamoyl β-dfucopyranoside (Jenisseensoside E) 3--[β-D-Galactopyranosyl-(1 2)-β-D-glucuronopyranosyl]quillaic acid-28--α- L-rhamnopyranosyl-(1 2)-3--acetyl-4--cis-p-methoxycinnamoyl β-dfucopyranoside (Jenisseensoside F) 3--[β-D-Galactopyranosyl-(1 2)-β-D-glucuronopyranosyl] quillaic acid-28--[α- L-arabinopyranosyl-(1 2)-α-L-arabinopyranosyl-(1 3)-β-D-xylopyranosyl- (1 4)-α-L-rhamnopyranosyl-(1 2)]-[6--acetyl-β-D-glucopyranosyl-(1 3)]-4- -acetyl-β-d-fucopyranoside 3--[β-D-Galactopyranosyl-(1 2)-β-D-glucuronopyranosyl]-28--[[α-L- arabinopyranosyl-(1 2)-α-L-arabinopyranosyl-(1 3)-β-D-xylopyranosyl-(1 4)- α-l-rhamnopyranosyl-(1 2)]-[β-D-glucopyranosyl-(1 3)]-4--acetyl-β-Dfucopyranosyl] quillaic acid 3--β-D-Galactopyranosyl(1 2)-β-D-glucuronopyranosyl-3β,16α-dihydroxy-23- oxoolean-12-en-28-oic acid 28--β-D-xylopyranosyl(1 4)-[β-Dglucopyranosyl(1 2)]-α-L-rhamnopyranosyl (1 2)-β-D-fucopyranoside (Silenosides B) R 1 = -β-d-glcuap-2 1-β-D-Galp R 2 = (3--Ac-, 4--E-p-methoxycinnamoyl-)-β-D-Fucp-2 1- α-l-rhap R 1 = -β-d-glcuap-2 1-β-D-Galp R 2 = (3--Ac-, 4--Z-p-methoxycinnamoyl-)-β-D-Fucp-2 1- α-l-rhap R 1 = -β-d-glcuap-2 1-β-D-Galp R 2 = 4--Ac-β-D-Fucp- R 1 = -β-d-glcuap-2 1-β-D-Galp R 2 = 4--Ac-β-D-Fucp- R 1 = -β-d-glcuap-2 1-β-D-Galp R 2 = -β-d-fucp-2 1- α-l-rhap- 3 1-(6--Ac)-β-D-Glcp 2 1-α-L-Rhap-4 1-β-D- Xylp-3 1-α-L-Arap-2 1- α-l-arap 3 1-β-D-Glcp 2 1-α-L-Rhap-4 1-β-D- Xylp-3 1-α-L-Arap-2 1- α-l-arap 2 1-β-D-Glcp 4 1-β-D-Xylp S. fortunei Wis [28] S. fortunei Wis [28] S. fortunei Wis [28] S. fortunei Wis [164] S. vulgaris (Moench) Garcke [157]
26 Diversity 2014, α-L-Arap 3--α-L-Arabinopyranosyl(1 3)-[β-D-galactopyranosyl (1 2)]-β-D- R 1 = -β-d-glcuap- glucuronopyranosyl-3β,16α-dihydroxy-23-oxoolean-12-en-28-oic acid 28--β-Dxylopyranosyl(1 4)-[β-D-glucopyranosyl(1 2)]-α-L-rhamnopyranosyl (1 2)-β- D-fucopyranoside R 2 = -β-d-fucp β-D-Galp 2 1-β-D-Glcp S. vulgaris (Moench) Garcke [157] (Silenosides C) α-l-rhap- 4 1-β-D-Xylp 2 1-β-D-Galp 3--{β-D-Galactopyranosyl-(1 2)-[β-D-xylopyranosyl]-(1 3)]-β-D- R 1 = -β-d-glcuap- glucuronopyranosyl}-28--{β-d-xylopyranosyl-(1 4)-α-L-rhamnopyranosyl- (1 2)]-[4,6-di--acetyl-β-D-glycopyranosyl-(1 3)]-4--acetyl-β-Dfucopyranosyl] quillaic acid 3 1-β-D-Xylp 2 1-α-L-Rhap-4 1-β-D- Xylp S. rubicunda Franch [158] (Silenorubicunoside B) R 2 = (4--Ac)-β-D-Fucp- 3 1-(4,6-di--Ac)-β-D- Glcp 2 1-β-D-Galp 3--β-D-Galactopyranosyl-(1 2)-[β-D-xylopyranosyl-(1 3)]-β-D- R 1 = -β-d-glcuap- glucuronopyrannosyl}-28--{β-d-xylopyranosyl-(1 4)-α-L-rhamnopyranosyl- (l 2)-[6--acetyl-β-D-glycopyranosyl-(1 3)]-4--acetyl -β-dfucopyranosyl}quillaic acid R 2 = (4--Ac)-β-D-Fucp- 3 1-β-D-Xylp 2 1-α-L-Rhap-4 1-β-D- Xylp S. rubicunda Franch [158] 3 1-(6--Ac)-β-D-Glcp
27 Diversity 2014, β-D-Galp 3--{β-D-Galactopyranosyl-(1 2)-[β-D-xylopyranosyl-(1 3)]-β-D- glucuronopyrannosyl}-28--{β-d-xylopyranosyl-(1 3)-β-D-xylopyranosyl-(1 4)- α-l-rhamnopyranosyl-(l 2)-[3,4-di--acetyl-β-D-quinovopyranosyl-(1 4)]-β-Dfucopyranosyl}quillaic acid (Pachystegioside A) R 1 = -β-d-glcuap- R 2 = -β-d-fucp- 3 1-β-D-Xylp 2 1-α-L-Rhap-4 1-β-D- Xylp-3 1-β-D-Xylp S. rubicunda Franch [158] 4 1-(3,4-di--Ac)-β-D- Quip 2 1-β-D-Galp Quillaic acid 3--β-xylopyranosyl-(1 3)-[β-galactopyranosyl-(1 2)]-βglucuronopyranside R 1 = -β-d-glcuap- 3 1-β-D-Xylp S. cucubalus Wib [159] R 2 = Quillaic acid 3--glucuronide R 1 = -β-d-glcuap R 2 = S. vulgaris (Moench) Garcke [161] 2 1-β-D-Galp R 1 = -β-d-glcuap- 3--β-D-Xylopyranosyl-(1 3)-β-D-galactopyranosyl-(1 2)-β-Dglucuronopyranosyl quillaic acid 28--β-L-rhamnopyranosyl-(1 2)-[4- methoxycinnamoyl-(3)]-4--acetyl-β-d-fucopyranoside (Silenoside) R 2 = (4--Ac)-β-D-Fucp- 3 1-β-D-Xylp 2 1-α-L-Rhap S. szechuensis F.N. Williams [165] 3 (4-methoxy cinnamoyl) 2 1-β-D-Galp 3--β-D-Galactopyranosyl(1 2)] [β-d-xylopyranosyl (1 3)]-6--butyl-β-Dglucuronopyranosyl quillaic acid 28--[α-L-rhamnopyranosyl(1 2)]-3--acetyl-4- -[(E)-4-methoxycinnamoyl]-β-D- fucopyranosyl ester (Visciduloside A) R 1 = 6--Bu-β-D-GlcUAp- 3 1-β-D-Xylp R 2 = 3--Ac-4--(E)-methoxycynnamoyl-β-D-Fucp-2 1-α-L-Rhap S. viscidula Franch [166]
28 Diversity 2014, β-D-Galp 3--β-D-Galactopyranosyl(1 2)] [β-d-xylopyranosyl (1 3)]-6--butyl-β-Dglucuronopyranosyl quillaic acid 28--[α-L-rhamnopyranosyl(1 2)]-3--acetyl-4- -[(Z)-4-methoxycinnamoyl]-β-D- fucopyranosyl ester (Visciduloside B) R 1 = 6--Bu-β-D-GlcUAp- 3 1-β-D-Xylp R 2 = 3--Ac-4--(Z)-4-methoxycynnamoyl-β-D-Fucp-2 1-α-L-Rhap S. viscidula Franch [166] R 1 = 3β,16α-Dihydroxyolean-12-en-23α,28β-dioic acid 28--{[α-D-mannopyranosyl- (1 4)][α-D-galactopyranosyl-(1 6)]-β-D-glycopyranosyl-(1 3)}[β-D-6--((3R)-3- hydroxy-3-methylglutaryl)glucopyranosyl-(1 6)-β-D-glucopyranoside (Silenoviscoside D) R 2 = -β-d-glcp- 3 1-β-D-Galp 6 1-β-D-6--(3--3- methylglytaryl)-β-d-glcp 4 1-α-D-Manp 6 1-α-D-Galp S. viscidula Franch [166] 2 1-β-D-Galp 3--[β-D-Galactopyranosyl(1 2)] [β-d-xylopyranosyl (1 3)]-[6--methyl-β-Dglucuronopyranosyl] quillaic acid 28--[α-L-rhamnopyranosyl(1 2)]-[3--acetyl-4- -(E)-para-methoxycinnamoyl-β-D-fucopyranosyl]ester (Sinocrassuloside VIII) R 1 = 6--Me-β-D-GlcUAp- 3 1-β-D-Xylp R 2 = 3--Ac-4--(E)-p-methoxycynnamoyl-β-D-Fucp-2 1- α-l-rhap S. viscidula Franch [166] 2 1-β-D-Galp 3--[β-D-Galactopyranosyl(1 2)] [β-d-xylopyranosyl (1 3)]-[6--methyl-β-Dglucuronopyranosyl] quillaic acid 28--[α-L-rhamnopyranosyl(1 2)]-[3--acetyl-4- -(Z)-para-methoxycinnamoyl-β-D-fucopyranosyl]ester (Sinocrassuloside IX) R 1 = 6--Me-β-D-GlcUAp- 3 1-β-D-Xylp R 2 = 3--Ac-4--(Z)-p-methoxycynnamoyl-β-D-Fucp-2 1- α-l-rhap S. viscidula Franch [166] 3--{β-D-Galactopyranosyl-(1 2)-[β-D-xylopyranosyl-(1 3)]-β-Dglucuronopyrannosyl}-28--{α-L-rhamnopyranosyl-(l 2)-4--(E)-pmethoxycinnamoyl-β-D-fucopyranosyl}quillaic acid (Sinocrassuloside X) R 1 = β-d-glcuap- 2 1-β-D-Galp 3 1-β-D-Xylp R 2 = 4--(E)-p-methoxycynnamoyl-β-D-Fucp-2 1-α-L-Rhap S. rubicunda Franch [158]
29 Diversity 2014, β-D-Galp 3--β-D-Galactopyranosyl-(1 2)-[β-D-xylopyranosyl-(1 3)]-β-D- R 1 = β-d-glcuap- glucuronopyranosyl quillaic acid 28--β-D-xylopyranosyl-(1 3)-β-D-xylopyranosyl- (1 4)-α-L-rhamnopyranosyl-(1 4)-[2 --acetyl-β-d-quinovopyranosyl-(1 2)]-3 - -acetyl-β-d-fucopyranoside R 2 = (3--Ac)-β-D-Fucp- 3 1-β-D-Xylp 2 1-(2--Ac)-β-D-Quip S. rubicunda Franch [167] (Rubicunoside A) 4 1-α-L-Rhap-4 1-β-D- Xylp-3 1-β-D-Xylp 2 1-β-D-Galp R 1 = -β-d-glcuap- R 2 = -(3--Ac)-β-D-Fucp- 3--[β-D-Galactopyranosyl-(1 2)-[β-D-xylopyranosyl-(1 3)]-β-Dglucuronopyranosyl quillaic acid 28--β-D-xylopyranosyl-(1 3)-β-D-xylopyranosyl- (1 4)-α-L-rhamnopyranosyl-(1 4)-[β-D-glucopyranosyl-(1 4 )-β-dquinovopyranosyl-(1 2)]-3 --acetyl-β-d-fucopyranoside (Rubicunoside B) 3 1-β-D-Xylp 2 1-β-D-Quip-4 1-β-D- Glcp S. rubicunda Franch [167] 4 1-α-L-Rhap-4 1-β-D- Xylp-3 1-β-D-Xylp 2 1-β-D-Galp R 1 = -β-d-glcuap- 3--β-D-Galactopyranosyl-(1 2)-[β-D-xylopyranosyl-(1 3)]-β-Dglucuronopyranosyl quillaic acid 28--β-D-xylopyranosyl-(1 4)-α-Lrhamnopyranosyl-(1 4)-[4 --acetyl-β-d-glucopyranosyl-(1 2)]-β-Dfucopyranoside 3 1-β-D-Xylp 2 1-(4--Ac)-β-D-Glcp S. rubicunda Franch [167] (Rubicunoside C) R 2 = -β-d-fucp- 4 1-α-L-Rhap-4 1-β-D- Xylp
Aristotle University of Thessaloniki School of Chemical Engineering Department of Organic Chemistry
 Aristotle University of Thessaloniki School of Chemical Engineering Department of Organic Chemistry Comparative study of valorization of pomegranate and wine wastes- Added value products and biological
Aristotle University of Thessaloniki School of Chemical Engineering Department of Organic Chemistry Comparative study of valorization of pomegranate and wine wastes- Added value products and biological
DEMETRIOS KOURETAS PROFESSOR DEPARTMENT OF BIOCHEMISTRY & BIOTECHNOLOGY UNIVERSITY OF THESSALY, GREECE
 DEMETRIOS KOURETAS PROFESSOR DEPARTMENT OF BIOCHEMISTRY & BIOTECHNOLOGY UNIVERSITY OF THESSALY, GREECE Entrepreneurial Discovery Focus Group on wine for Eastern Macedonia and Thrace Drama, Greece Vitis
DEMETRIOS KOURETAS PROFESSOR DEPARTMENT OF BIOCHEMISTRY & BIOTECHNOLOGY UNIVERSITY OF THESSALY, GREECE Entrepreneurial Discovery Focus Group on wine for Eastern Macedonia and Thrace Drama, Greece Vitis
COCKLES & CAMPION STUDY
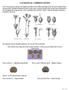 COCKLES & CAMPION STUDY In the Caryophyllaceae family, seed shapes and surface textures differ depending on how the seed is dispersed from the parent plant. Methods of dispersal include by wind, water,
COCKLES & CAMPION STUDY In the Caryophyllaceae family, seed shapes and surface textures differ depending on how the seed is dispersed from the parent plant. Methods of dispersal include by wind, water,
ISSN: Selangor, Malaysia. *Corresponding author
 Research Article ISSN:2230-7346 Journal of Global Trends in Pharmaceutical Sciences Vol.2, Issue 4, pp -404-410, October December 2011 ANTIMICROBIAL ACTIVITY AND PHYTOCHEMICAL ANALYSIS OF NELUMBO NUCIFERA
Research Article ISSN:2230-7346 Journal of Global Trends in Pharmaceutical Sciences Vol.2, Issue 4, pp -404-410, October December 2011 ANTIMICROBIAL ACTIVITY AND PHYTOCHEMICAL ANALYSIS OF NELUMBO NUCIFERA
ORIENTAL TEA COMPLEX. Product for anti-aging. the one who knows natural products
 Product for anti-aging Head Office #B-902, Digital Empire, 980-3, Youngtong-dong, Youngtong-gu, Suwon, Gyeonggi-do, Korea Tel: +82.31.303.5240 Fax: +82.31.303.5245 Factory #504, 158B 11L, 730-10, Gojan-dong,
Product for anti-aging Head Office #B-902, Digital Empire, 980-3, Youngtong-dong, Youngtong-gu, Suwon, Gyeonggi-do, Korea Tel: +82.31.303.5240 Fax: +82.31.303.5245 Factory #504, 158B 11L, 730-10, Gojan-dong,
Peppermint Tea (Bags)
 Peppermint Tea (Bags) Dried peppermint leaves make a minty, refreshing drink that is highly satisfying both hot and cold. A native of the Mediterranean, peppermint leaves were often used to crown luminaries
Peppermint Tea (Bags) Dried peppermint leaves make a minty, refreshing drink that is highly satisfying both hot and cold. A native of the Mediterranean, peppermint leaves were often used to crown luminaries
Pink flower. Water lily. Cosmos. Prunus Mume Flower
 PINK FLOWER Complex Pink flower Prunus Mume Flower Water lily Cosmos Rose Camellia Lotus Flower Japanese apricot s flower Common name Latin name INCI name Efficacy Mume Flos Prunus mume Siebold & Zucc.
PINK FLOWER Complex Pink flower Prunus Mume Flower Water lily Cosmos Rose Camellia Lotus Flower Japanese apricot s flower Common name Latin name INCI name Efficacy Mume Flos Prunus mume Siebold & Zucc.
CHAPTER 4 ISOLATION OF ANTIFUNGAL COMPOUNDS FROM C. dentata (Burm.f) C.A. Sm.
 CHAPTER 4 ISOLATION OF ANTIFUNGAL COMPOUNDS FROM C. dentata (Burm.f) C.A. Sm. 4.1. INTRODUCTION 4.1.1. Compounds isolated from Cornaceae family Reports concerning isolation of compounds from Curtisia dentata
CHAPTER 4 ISOLATION OF ANTIFUNGAL COMPOUNDS FROM C. dentata (Burm.f) C.A. Sm. 4.1. INTRODUCTION 4.1.1. Compounds isolated from Cornaceae family Reports concerning isolation of compounds from Curtisia dentata
Effect of Different Levels of Grape Pomace on Blood Serum Biochemical Parameters Broiler Chicks at 29 and 49 days of age
 Effect of Different Levels of Grape Pomace on Blood Serum Biochemical Parameters Broiler Chicks at 29 and 49 days of age Safdar Dorri * (1), Sayed Ali Tabeidian (2), majid Toghyani (2), Rahman Jahanian
Effect of Different Levels of Grape Pomace on Blood Serum Biochemical Parameters Broiler Chicks at 29 and 49 days of age Safdar Dorri * (1), Sayed Ali Tabeidian (2), majid Toghyani (2), Rahman Jahanian
 CHINA LISTED LIGHTENING TESTED SKIN-LIGHTENING EFFECT FROM PLANT EXTRACTS lightening activity. lightening lightening 5 4. Saija A, et al., in vitro antioxidant activity and in vivo photoprotective effect
CHINA LISTED LIGHTENING TESTED SKIN-LIGHTENING EFFECT FROM PLANT EXTRACTS lightening activity. lightening lightening 5 4. Saija A, et al., in vitro antioxidant activity and in vivo photoprotective effect
Effect of Different Levels of Grape Pomace on Performance Broiler Chicks
 Effect of Different Levels of Grape Pomace on Performance Broiler Chicks Safdar Dorri * (1), Sayed Ali Tabeidian (2), majid Toghyani (2), Rahman Jahanian (3), Fatemeh Behnamnejad (1) (1) M.Sc Student,
Effect of Different Levels of Grape Pomace on Performance Broiler Chicks Safdar Dorri * (1), Sayed Ali Tabeidian (2), majid Toghyani (2), Rahman Jahanian (3), Fatemeh Behnamnejad (1) (1) M.Sc Student,
Step 1: Brownie batter was prepared for each oil variation following the recipe on the Betty Crocker brownie mix box.
 Title: The effects of substituting coconut oil in brownies Abstract: In baking brownies, canola oil was replaced with coconut oil in the same amount to test the effect on texture, flavor and overall satisfaction.
Title: The effects of substituting coconut oil in brownies Abstract: In baking brownies, canola oil was replaced with coconut oil in the same amount to test the effect on texture, flavor and overall satisfaction.
Perfect Grape. What s so special about Muscadine Grapes?
 The is a powerful whole food supplement, made from the Seed, Skin and Pulp of Muscadine Grapes. With its high levels of Resveratrol and other antioxidants, The has been shown to help promote superior health
The is a powerful whole food supplement, made from the Seed, Skin and Pulp of Muscadine Grapes. With its high levels of Resveratrol and other antioxidants, The has been shown to help promote superior health
Mulberry Assorted. Morus rubra, Morus alba, Morus nigra. (a) Morus rubra red mulberry. Female flowers. Male flowers. (b) Morus alba white mulberry
 Mulberry Assorted Morus rubra, Morus alba, Morus nigra (a) Morus rubra red mulberry Female flowers (b) Morus alba white mulberry Male flowers (c) Morus nigra black mulberry Female flower Common names Origin
Mulberry Assorted Morus rubra, Morus alba, Morus nigra (a) Morus rubra red mulberry Female flowers (b) Morus alba white mulberry Male flowers (c) Morus nigra black mulberry Female flower Common names Origin
Influence of yeast strain choice on the success of Malolactic fermentation. Nichola Hall Ph.D. Wineries Unlimited, Richmond VA March 29 th 2012
 Influence of yeast strain choice on the success of Malolactic fermentation Nichola Hall Ph.D. Wineries Unlimited, Richmond VA March 29 th 2012 INTRODUCTION Changing conditions dictate different microbial
Influence of yeast strain choice on the success of Malolactic fermentation Nichola Hall Ph.D. Wineries Unlimited, Richmond VA March 29 th 2012 INTRODUCTION Changing conditions dictate different microbial
Determination of caffeine content in tea and soft drink. BCH445 [Practical] 1
![Determination of caffeine content in tea and soft drink. BCH445 [Practical] 1 Determination of caffeine content in tea and soft drink. BCH445 [Practical] 1](/thumbs/95/124814804.jpg) Determination of caffeine content in tea and soft drink BCH445 [Practical] 1 Caffeine, the common name for 1,3,7-trimethylxanthine. It belongs to a group of methylxanthene. 2 Caffeine is a chemical that
Determination of caffeine content in tea and soft drink BCH445 [Practical] 1 Caffeine, the common name for 1,3,7-trimethylxanthine. It belongs to a group of methylxanthene. 2 Caffeine is a chemical that
Analysing the shipwreck beer
 Analysing the shipwreck beer Annika Wilhelmson, John Londesborough and Riikka Juvonen VTT Technical Research Centre of Finland Press conference 10 th May 2012 2 The aim of the research was to find out
Analysing the shipwreck beer Annika Wilhelmson, John Londesborough and Riikka Juvonen VTT Technical Research Centre of Finland Press conference 10 th May 2012 2 The aim of the research was to find out
L-Theanine Clinical Studies
 ALL A B C D E F G I K L M N O P Q R S T V Z L-Theanine Clinical Studies Nippon Nogei Kagakukaishi. Kobayashi K, et al. Effects of L-theanine on the release of a- brain waves in human volunteers. 1998;72(2):153-7.
ALL A B C D E F G I K L M N O P Q R S T V Z L-Theanine Clinical Studies Nippon Nogei Kagakukaishi. Kobayashi K, et al. Effects of L-theanine on the release of a- brain waves in human volunteers. 1998;72(2):153-7.
Effects of ginger on the growth of Escherichia coli
 Effects of ginger on the growth of Escherichia coli Jennes Eloïse Klapp Vanessa Project Jonk Fuerscher 2014 Effects of ginger on the growth of Escherichia Coli Jennes Eloïse Klapp Vanessa Abstract The
Effects of ginger on the growth of Escherichia coli Jennes Eloïse Klapp Vanessa Project Jonk Fuerscher 2014 Effects of ginger on the growth of Escherichia Coli Jennes Eloïse Klapp Vanessa Abstract The
Antibacterial Activity Of Camellia Sinsnsis Against Dental Caries: Comparison Of Antibacterial Activity Of Water And Ethanol Extracts Of Camellia
 Antibacterial Activity Of Camellia Sinsnsis Against Dental Caries: Comparison Of Antibacterial Activity Of Water And Ethanol Extracts Of Camellia Sinsnsis Against Dental Caries By Arifa Tahir If searched
Antibacterial Activity Of Camellia Sinsnsis Against Dental Caries: Comparison Of Antibacterial Activity Of Water And Ethanol Extracts Of Camellia Sinsnsis Against Dental Caries By Arifa Tahir If searched
The miraculous power of Bulgarian yogurt. Created by LB BULGARICUM
 The miraculous power of Bulgarian yogurt HISTORY REMARKS Its secret is hidden in its micro-flora and the specific combination of strains from two species - Lactobacillus bulgaricus and Streptococcus thermophilus
The miraculous power of Bulgarian yogurt HISTORY REMARKS Its secret is hidden in its micro-flora and the specific combination of strains from two species - Lactobacillus bulgaricus and Streptococcus thermophilus
The Effect of Green Tea on the Texture, Taste and Moisture of Gharidelli Double Chocolate Brownies
 Katie Mitsch Madison Moore FN 453 The Effect of Green Tea on the Texture, Taste and Moisture of Gharidelli Double Chocolate Brownies Introduction: The Center for Disease Control states that cancer and
Katie Mitsch Madison Moore FN 453 The Effect of Green Tea on the Texture, Taste and Moisture of Gharidelli Double Chocolate Brownies Introduction: The Center for Disease Control states that cancer and
INDICE. - Gold Imperial - Gold Imperial Rosé - Gold Imperial Blue.
 INDICE - Gold Imperial - Gold Imperial Rosé - Gold Imperial Blue www.vinafragrance.eu BRUT GRAN RESERVA Viña Fragrance D'or Brut Reserve is a sparkling wine from natural Macabeo, Xarel'lo, Parellada and
INDICE - Gold Imperial - Gold Imperial Rosé - Gold Imperial Blue www.vinafragrance.eu BRUT GRAN RESERVA Viña Fragrance D'or Brut Reserve is a sparkling wine from natural Macabeo, Xarel'lo, Parellada and
Goji - the Oriental fruit of God
 Goji - the Oriental fruit of God Goji is the ripened fruit of Ningxia area in China. Goji is rich in polysaccharide, fat, protein, amino acid, taurine, betaine, vitamin B 1, vitamin B 2, vitamin E, vitamin
Goji - the Oriental fruit of God Goji is the ripened fruit of Ningxia area in China. Goji is rich in polysaccharide, fat, protein, amino acid, taurine, betaine, vitamin B 1, vitamin B 2, vitamin E, vitamin
Maurya Shalini 1, Dubey Prakash Ritu 2 Research Scholar 1, Associate Professor 2 Ethelind College of Home Science, SHUATS Allahabad, U.P.
 PHYSICO- CHEMICAL PROPERTIES OF ANTIOXIDANT RICH HEALTHY BEVERAGES PREPARED BY USING PINEAPPLE JUICE AND GUAVA LEAVES EXTRACTS FLAVOURED WITH HERABS (MINT AND BASIL) Maurya Shalini 1, Dubey Prakash Ritu
PHYSICO- CHEMICAL PROPERTIES OF ANTIOXIDANT RICH HEALTHY BEVERAGES PREPARED BY USING PINEAPPLE JUICE AND GUAVA LEAVES EXTRACTS FLAVOURED WITH HERABS (MINT AND BASIL) Maurya Shalini 1, Dubey Prakash Ritu
Phytochemical Screening and Antimicrobial Properties of a Common Brand of Black Tea (Camellia sinensis) Marketed in Nigerian Environment
 Advanced Pharmaceutical Bulletin, 2012, 2(2), 259-263 doi: 10.5681/apb.2012.040 http://apb.tbzmed.ac.ir/ Phytochemical Screening and Antimicrobial Properties of a Common Brand of Black Tea (Camellia sinensis)
Advanced Pharmaceutical Bulletin, 2012, 2(2), 259-263 doi: 10.5681/apb.2012.040 http://apb.tbzmed.ac.ir/ Phytochemical Screening and Antimicrobial Properties of a Common Brand of Black Tea (Camellia sinensis)
Enzymatic Hydrolysis of Ovomucin and the Functional and Structural Characteristics of Peptides in the Hydrolysates
 Animal Industry Report AS 663 ASL R3128 2017 Enzymatic Hydrolysis of Ovomucin and the Functional and Structural Characteristics of Peptides in the Hydrolysates Sandun Abeyrathne Iowa State University Hyun
Animal Industry Report AS 663 ASL R3128 2017 Enzymatic Hydrolysis of Ovomucin and the Functional and Structural Characteristics of Peptides in the Hydrolysates Sandun Abeyrathne Iowa State University Hyun
Recovery of Health- Promoting Proanthocyanidins from Berry Co- Products by Alkalization
 Recovery of Health- Promoting Proanthocyanidins from Berry Co- Products by Alkalization Luke Howard Brittany White Ron Prior University of Arkansas, Department of Food Science Berry Health Benefits Symposium
Recovery of Health- Promoting Proanthocyanidins from Berry Co- Products by Alkalization Luke Howard Brittany White Ron Prior University of Arkansas, Department of Food Science Berry Health Benefits Symposium
Citrus Fruit Antimicrobial Effects. By John Seabrooke Central Catholic High School Grade 9
 Citrus Fruit Antimicrobial Effects By John Seabrooke Central Catholic High School Grade 9 Antimicrobials Natural Tea tree oil Onion Lemon juice Grapefruit seed extract Cinnamon Artificial Antibiotics Bleach
Citrus Fruit Antimicrobial Effects By John Seabrooke Central Catholic High School Grade 9 Antimicrobials Natural Tea tree oil Onion Lemon juice Grapefruit seed extract Cinnamon Artificial Antibiotics Bleach
1. Quinoa is Incredibly Nutritious
 Quinoa is the world s most popular superfood. It is loaded with protein, fiber and minerals, but doesn t contain any gluten. Here are 11 proven health benefits of quinoa. 1. Quinoa is Incredibly Nutritious
Quinoa is the world s most popular superfood. It is loaded with protein, fiber and minerals, but doesn t contain any gluten. Here are 11 proven health benefits of quinoa. 1. Quinoa is Incredibly Nutritious
Author's response to reviews
 Author's response to reviews Title: Coffee bean extracts rich and poor in kahweol both give rise to elevation of liver enzymes in healthy volunteers Authors: Mr Mark V Boekschoten (Mark.Boekschoten@wur.nl)
Author's response to reviews Title: Coffee bean extracts rich and poor in kahweol both give rise to elevation of liver enzymes in healthy volunteers Authors: Mr Mark V Boekschoten (Mark.Boekschoten@wur.nl)
ImuPro shows you the way to the right food for you. And your path for better health.
 Your personal ImuPro Screen + documents Sample ID: 33333 Dear, With this letter, you will receive the ImuPro result for your personal IgG food allergy test. This laboratory report contains your results
Your personal ImuPro Screen + documents Sample ID: 33333 Dear, With this letter, you will receive the ImuPro result for your personal IgG food allergy test. This laboratory report contains your results
European Union comments for the. CODEX COMMITTEE ON CONTAMINANTS IN FOOD (CCCF) 4th Session. Izmir, Turkey, April 2010.
 European Union comments for the 13.04. 2010 CODEX COMMITTEE ON CONTAMINANTS IN FOOD (CCCF) 4th Session Izmir, Turkey, 26 30 April 2010 Agenda Item 5 Proposed Draft Maximum Levels for Melamine in Food and
European Union comments for the 13.04. 2010 CODEX COMMITTEE ON CONTAMINANTS IN FOOD (CCCF) 4th Session Izmir, Turkey, 26 30 April 2010 Agenda Item 5 Proposed Draft Maximum Levels for Melamine in Food and
Wine anthocyanins: gut metabolism key to anti-cancer effects?
 Wine anthocyanins: gut metabolism key to anti-cancer effects? Andrew Waterhouse Viticulture and Enology Hilo, April 28, 2011 Modern History-Vin et Santé St. Leger et al. Factors associated with cardiac
Wine anthocyanins: gut metabolism key to anti-cancer effects? Andrew Waterhouse Viticulture and Enology Hilo, April 28, 2011 Modern History-Vin et Santé St. Leger et al. Factors associated with cardiac
MATURITY AND RIPENING PROCESS MATURITY
 MATURITY AND RIPENING PROCESS MATURITY It is the stage of fully development of tissue of fruit and vegetables only after which it will ripen normally. During the process of maturation the fruit receives
MATURITY AND RIPENING PROCESS MATURITY It is the stage of fully development of tissue of fruit and vegetables only after which it will ripen normally. During the process of maturation the fruit receives
L-Theanine: How a Unique Anxiety Reducer and Mood Enhancer Increases Alpha Waves and Alertness
 L-Theanine: How a Unique Anxiety Reducer and Mood Enhancer Increases Alpha Waves and Alertness by Carolyn Perrini, CLS, CNC Hundreds of studies exist showing the many health benefits of green tea. But
L-Theanine: How a Unique Anxiety Reducer and Mood Enhancer Increases Alpha Waves and Alertness by Carolyn Perrini, CLS, CNC Hundreds of studies exist showing the many health benefits of green tea. But
Presented by: Grant Washing Smith Director Anagenix Ltd.
 Presented by: Grant Washing Smith Director Anagenix Ltd. Traceability and Authenticity Growing Region Production and Manufacture What makes Actazin Unique? Actazin has a Four-Way action on gut health.
Presented by: Grant Washing Smith Director Anagenix Ltd. Traceability and Authenticity Growing Region Production and Manufacture What makes Actazin Unique? Actazin has a Four-Way action on gut health.
Sequential Separation of Lysozyme, Ovomucin, Ovotransferrin and Ovalbumin from Egg White
 AS 662 ASL R3104 2016 Sequential Separation of Lysozyme, Ovomucin, Ovotransferrin and Ovalbumin from Egg White Sandun Abeyrathne Iowa State University Hyunyong Lee Iowa State University, hdragon@iastate.edu
AS 662 ASL R3104 2016 Sequential Separation of Lysozyme, Ovomucin, Ovotransferrin and Ovalbumin from Egg White Sandun Abeyrathne Iowa State University Hyunyong Lee Iowa State University, hdragon@iastate.edu
Varietal Specific Barrel Profiles
 RESEARCH Varietal Specific Barrel Profiles Beaulieu Vineyard and Sea Smoke Cellars 2006 Pinot Noir Domenica Totty, Beaulieu Vineyard Kris Curran, Sea Smoke Cellars Don Shroerder, Sea Smoke Cellars David
RESEARCH Varietal Specific Barrel Profiles Beaulieu Vineyard and Sea Smoke Cellars 2006 Pinot Noir Domenica Totty, Beaulieu Vineyard Kris Curran, Sea Smoke Cellars Don Shroerder, Sea Smoke Cellars David
Dioxins&Furans 12 F 1 11 A 2 10 Q s
 Dioxins & Furans 12 10 11 9 F 8 A s 1 3 2 4 7 5 1 Copyright Toxics Link, 2013. All rights reserved. Dioxins & Furans Frequently Asked uestions Published in 2013 by Toxics Link Toxics Link H-2, Jangpura
Dioxins & Furans 12 10 11 9 F 8 A s 1 3 2 4 7 5 1 Copyright Toxics Link, 2013. All rights reserved. Dioxins & Furans Frequently Asked uestions Published in 2013 by Toxics Link Toxics Link H-2, Jangpura
III InTIfir IIII A COMPARATIVE STUDY OF BLACK TEA AND INSTANT TEA TO DEVELOP AN INSTANT TEA TABLE~ WITH RETAINED HEALTH PROMOTING PROPERTIES
 A COMPARATIVE STUDY OF BLACK TEA AND INSTANT TEA TO DEVELOP AN INSTANT TEA TABLE~ WITH RETAINED HEALTH PROMOTING PROPERTIES By PALAMANDADIGE THARANGI SRIYANGlKA RAJAPAKSHA MUDALIGE Thesis submitted to
A COMPARATIVE STUDY OF BLACK TEA AND INSTANT TEA TO DEVELOP AN INSTANT TEA TABLE~ WITH RETAINED HEALTH PROMOTING PROPERTIES By PALAMANDADIGE THARANGI SRIYANGlKA RAJAPAKSHA MUDALIGE Thesis submitted to
Lycopene is a 40 carbon atom open chain polyisoprenoid with 11 conjugated double bonds. The structural formula of lycopene is represented as follows:
 Lycopene is a 40 carbon atom open chain polyisoprenoid with 11 conjugated double bonds. The structural formula of lycopene is represented as follows: Many factors could affect the lycopene concentration
Lycopene is a 40 carbon atom open chain polyisoprenoid with 11 conjugated double bonds. The structural formula of lycopene is represented as follows: Many factors could affect the lycopene concentration
Preliminary Studies on the Preservation of Longan Fruit in Sugar Syrup
 Universities Research Journal 2011, Vol. 4, No. 3 Preliminary Studies on the Preservation of Longan Fruit in Sugar Syrup Khin Hla Mon Abstract This research work was emphasized on the preservation of longan
Universities Research Journal 2011, Vol. 4, No. 3 Preliminary Studies on the Preservation of Longan Fruit in Sugar Syrup Khin Hla Mon Abstract This research work was emphasized on the preservation of longan
Preventive and curative efficacy of Ostrinil against the Palm Borer Paysandisia archon (Burmeister, 1880)
 Preventive and curative efficacy of Ostrinil against the Palm Borer Paysandisia archon (Burmeister, 1880) Samantha BESSE Natural Plant Protection Studies realized in partnership with 2 protagonists Context
Preventive and curative efficacy of Ostrinil against the Palm Borer Paysandisia archon (Burmeister, 1880) Samantha BESSE Natural Plant Protection Studies realized in partnership with 2 protagonists Context
Effects of Leaf Removal and UV-B on Flavonoids, Amino Acids and Methoxypyrazines
 Effects of Leaf Removal and UV-B on Flavonoids, Amino Acids and Methoxypyrazines Professor Brian Jordan Centre for Viticulture & Oenology, Lincoln University What are the major factors to be considered
Effects of Leaf Removal and UV-B on Flavonoids, Amino Acids and Methoxypyrazines Professor Brian Jordan Centre for Viticulture & Oenology, Lincoln University What are the major factors to be considered
Decolorisation of Cashew Leaves Extract by Activated Carbon in Tea Bag System for Using in Cosmetics
 International Journal of Sciences Research Article (ISSN 235-3925) Volume 1, Issue Oct 212 http://www.ijsciences.com Decolorisation of Cashew Leaves Extract by Activated Carbon in Tea Bag System for Using
International Journal of Sciences Research Article (ISSN 235-3925) Volume 1, Issue Oct 212 http://www.ijsciences.com Decolorisation of Cashew Leaves Extract by Activated Carbon in Tea Bag System for Using
Isolation and characterization of endophytic bacteria of coffee plants and their potential in caffeine degradation
 Environmental Toxicology 293 Isolation and characterization of endophytic bacteria of coffee plants and their potential in caffeine degradation F. V. Nunes & I. S. de Melo Laboratory o f Environm ental
Environmental Toxicology 293 Isolation and characterization of endophytic bacteria of coffee plants and their potential in caffeine degradation F. V. Nunes & I. S. de Melo Laboratory o f Environm ental
EFFECT OF SOME TECHNOLOGICAL FACTORS ON THE CONTENT OF ACETALDEHYDE IN BEER
 Studii şi Cercetări Ştiinţifice Chimie şi Inginerie Chimică, Biotehnologii, Industrie Alimentară Scientific Study & Research Chemistry & Chemical Engineering, Biotechnology, Food Industry 2010, 11 (3),
Studii şi Cercetări Ştiinţifice Chimie şi Inginerie Chimică, Biotehnologii, Industrie Alimentară Scientific Study & Research Chemistry & Chemical Engineering, Biotechnology, Food Industry 2010, 11 (3),
Audrey Page. Brooke Sacksteder. Kelsi Buckley. Title: The Effects of Black Beans as a Flour Replacer in Brownies. Abstract:
 Audrey Page Brooke Sacksteder Kelsi Buckley Title: The Effects of Black Beans as a Flour Replacer in Brownies Abstract: One serving of beans can provide 30% of an average adult s daily recommendation for
Audrey Page Brooke Sacksteder Kelsi Buckley Title: The Effects of Black Beans as a Flour Replacer in Brownies Abstract: One serving of beans can provide 30% of an average adult s daily recommendation for
AN ENOLOGY EXTENSION SERVICE QUARTERLY PUBLICATION
 The Effects of Pre-Fermentative Addition of Oenological Tannins on Wine Components and Sensorial Qualities of Red Wine FBZDF Wine. What Where Why How 2017 2. October, November, December What the authors
The Effects of Pre-Fermentative Addition of Oenological Tannins on Wine Components and Sensorial Qualities of Red Wine FBZDF Wine. What Where Why How 2017 2. October, November, December What the authors
ANTIMICROBIAL ACTIVITY OF WHITE AND PINK NELUMBO NUCIFERA GAERTN FLOWERS D.BRINDHA*, D.ARTHI. For author affiliations, see end of text
 ANTIMICROBIAL ACTIVITY OF WHITE AND PINK NELUMBO NUCIFERA GAERTN FLOWERS D.BRINDHA*, D.ARTHI For author affiliations, see end of text ABSTRACT Nelumbo nucifera Gaertn (Family: Nelumbonaceae), medicinally
ANTIMICROBIAL ACTIVITY OF WHITE AND PINK NELUMBO NUCIFERA GAERTN FLOWERS D.BRINDHA*, D.ARTHI For author affiliations, see end of text ABSTRACT Nelumbo nucifera Gaertn (Family: Nelumbonaceae), medicinally
Melamine and Analogues in Food
 Melamine and Analogues in Food Yan Gu Office of Food Additive Safety Center of Food Safety and Applied Nutrition Food and Drug Administration April 23, 2009 1 Toxicology of Melamine and Analogues Sources
Melamine and Analogues in Food Yan Gu Office of Food Additive Safety Center of Food Safety and Applied Nutrition Food and Drug Administration April 23, 2009 1 Toxicology of Melamine and Analogues Sources
Preventing Salmonella Contamination of Peanut Products. Michael Doyle
 Preventing Salmonella Contamination of Peanut Products Michael Doyle Sources of Salmonella Contamination Primary sources of salmonellae are intestinal tracts of animals (domestic and wild) and humans;
Preventing Salmonella Contamination of Peanut Products Michael Doyle Sources of Salmonella Contamination Primary sources of salmonellae are intestinal tracts of animals (domestic and wild) and humans;
Technical Activities Focused On Reorganization of Federal Culture Collection of Pathogen Microorganisms
 State Research Center for Applied Microbiology and Biotechnology Technical Activities Focused On Reorganization of Federal Culture Collection of Pathogen Microorganisms Baranov A.M., Dunaitsev I. A., Dyatlov
State Research Center for Applied Microbiology and Biotechnology Technical Activities Focused On Reorganization of Federal Culture Collection of Pathogen Microorganisms Baranov A.M., Dunaitsev I. A., Dyatlov
Dr.Nibras Nazar. Microbial Biomass Production: Bakers yeast
 Microbial biomass In a few instances the cells i.e. biomass of microbes, has industrial application as listed in Table 3. The prime example is the production of single cell proteins (SCP) which are in
Microbial biomass In a few instances the cells i.e. biomass of microbes, has industrial application as listed in Table 3. The prime example is the production of single cell proteins (SCP) which are in
STUDIES ON THE CHROMATIC CHARACTERISTICS OF RED WINES AND COLOR EVOLUTION DURING MATURATION
 Scientific Bulletin. Series F. Biotechnologies, Vol. XVII, 2013 ISSN 2285-1364, CD-ROM ISSN 2285-5521, ISSN Online 2285-1372, ISSN-L 2285-1364 STUDIES ON THE CHROMATIC CHARACTERISTICS OF RED WINES AND
Scientific Bulletin. Series F. Biotechnologies, Vol. XVII, 2013 ISSN 2285-1364, CD-ROM ISSN 2285-5521, ISSN Online 2285-1372, ISSN-L 2285-1364 STUDIES ON THE CHROMATIC CHARACTERISTICS OF RED WINES AND
TESTING WINE STABILITY fining, analysis and interpretation
 TESTING WINE STABILITY fining, analysis and interpretation Carien Coetzee Stephanie Steyn FROM TANK TO BOTTLE Enartis Stabilisation School Testing wine stability Hazes/colour/precipitate Oxidation Microbial
TESTING WINE STABILITY fining, analysis and interpretation Carien Coetzee Stephanie Steyn FROM TANK TO BOTTLE Enartis Stabilisation School Testing wine stability Hazes/colour/precipitate Oxidation Microbial
Emerging Foodborne Pathogens with Potential Significance to the Middle East
 Emerging Foodborne Pathogens with Potential Significance to the Middle East Ahmed E. Yousef Department of Food Science and Technology (and Department of Microbiology) The Ohio State University Columbus,
Emerging Foodborne Pathogens with Potential Significance to the Middle East Ahmed E. Yousef Department of Food Science and Technology (and Department of Microbiology) The Ohio State University Columbus,
FUNCTIONAL PROPERTIES OF FLOURS PREPARED FROM GLUCOSINOLATE RICH VEGETABLES: ALUGBATI (Basella rubra)
 FUNCTIONAL PROPERTIES OF FLOURS PREPARED FROM GLUCOSINOLATE RICH VEGETABLES: ALUGBATI (Basella rubra) Janica Charelle S. Borja 1, Dominique S. Sedano 1 and Marissa G. Noel 1 1 Chemistry Department, De
FUNCTIONAL PROPERTIES OF FLOURS PREPARED FROM GLUCOSINOLATE RICH VEGETABLES: ALUGBATI (Basella rubra) Janica Charelle S. Borja 1, Dominique S. Sedano 1 and Marissa G. Noel 1 1 Chemistry Department, De
A new approach to understand and control bitter pit in apple
 FINAL PROJECT REPORT WTFRC Project Number: AP-07-707 Project Title: PI: Organization: A new approach to understand and control bitter pit in apple Elizabeth Mitcham University of California Telephone/email:
FINAL PROJECT REPORT WTFRC Project Number: AP-07-707 Project Title: PI: Organization: A new approach to understand and control bitter pit in apple Elizabeth Mitcham University of California Telephone/email:
Technical note. How much do potential precursor compounds contribute to reductive aromas in wines post-bottling?
 Technical note How much do potential precursor compounds contribute to reductive aromas in wines post-bottling? Introduction The formation of unpleasant reductive aromas in wines is an issue of concern
Technical note How much do potential precursor compounds contribute to reductive aromas in wines post-bottling? Introduction The formation of unpleasant reductive aromas in wines is an issue of concern
In This Issue: YOUR FRIENDLY NEIGHBORHOOD PHARMA- CY WITH MODERN TECHNOLOGY. Serving Your Loved Ones. Compounding with a heart.
 Gloyer s Pharmacy & Gifts Newsletter Vol 1 Issue 7 June 2015 July 2015 YOUR FRIENDLY NEIGHBORHOOD PHARMA- CY WITH MODERN TECHNOLOGY Thank you for subscribing to Gloyer s Monthly, a newsletter purposed
Gloyer s Pharmacy & Gifts Newsletter Vol 1 Issue 7 June 2015 July 2015 YOUR FRIENDLY NEIGHBORHOOD PHARMA- CY WITH MODERN TECHNOLOGY Thank you for subscribing to Gloyer s Monthly, a newsletter purposed
Pulverization of coffee silverskin extract as a source of antioxidant
 IOP Conference Series: Materials Science and Engineering PAPER OPEN ACCESS Pulverization of coffee silverskin extract as a source of antioxidant To cite this article: S Tan et al 216 IOP Conf. Ser.: Mater.
IOP Conference Series: Materials Science and Engineering PAPER OPEN ACCESS Pulverization of coffee silverskin extract as a source of antioxidant To cite this article: S Tan et al 216 IOP Conf. Ser.: Mater.
Nattokinase(Powder of extract natto culture mixture)for health foods
 Nattokinase(Powder of extract natto culture mixture)for health foods S. Takagaki 1), T. Ito 1), Y. Yanagisawa 2), H. Sumi 3) 1) Organo Food Tech Corporation 2) Chiba Institute of Science 3) Kurashiki University
Nattokinase(Powder of extract natto culture mixture)for health foods S. Takagaki 1), T. Ito 1), Y. Yanagisawa 2), H. Sumi 3) 1) Organo Food Tech Corporation 2) Chiba Institute of Science 3) Kurashiki University
Emerging Threats to Groundwater and Surface Water
 Emerging Threats to Groundwater and Surface Water Water Arabia 2009 Rick McGregor Industrial Supplies Center Background Big Blue River STP, Kanas City 1976 Germany, early 1990 s Stan & Linkerhäger, 1992
Emerging Threats to Groundwater and Surface Water Water Arabia 2009 Rick McGregor Industrial Supplies Center Background Big Blue River STP, Kanas City 1976 Germany, early 1990 s Stan & Linkerhäger, 1992
RESOLUTION OIV-OENO MONOGRAPH ON GLUTATHIONE
 RESOLUTION OIV-OENO 571-2017 MONOGRAPH ON GLUTATHIONE THE GENERAL ASSEMBLY, IN VIEW OF Article 2, paragraph 2 iv of the Agreement of 3 April 2001 establishing the International Organisation of Vine and
RESOLUTION OIV-OENO 571-2017 MONOGRAPH ON GLUTATHIONE THE GENERAL ASSEMBLY, IN VIEW OF Article 2, paragraph 2 iv of the Agreement of 3 April 2001 establishing the International Organisation of Vine and
How Much Sugar Is in Your Favorite Drinks?
 Lesson 3 How Much Sugar Is in Your Favorite Drinks? Objectives Students will: identify important nutrition information on beverages labels* perform calculations using nutrition information on beverages
Lesson 3 How Much Sugar Is in Your Favorite Drinks? Objectives Students will: identify important nutrition information on beverages labels* perform calculations using nutrition information on beverages
Bansode.D.S et al. IRJP 2012, 3 (11) INTERNATIONAL RESEARCH JOURNAL OF PHARMACY
 INTERNATIONAL RESEARCH JOURNAL OF PHARMACY www.irjponline.com ISSN 2230 8407 Research Article STUDIES ON ANTIMICROBIAL ACTIVITY AND PHYTOCHEMICAL ANALYSIS OF CITRUS FRUIT JUICES AGAINST SELECTED ENTERIC
INTERNATIONAL RESEARCH JOURNAL OF PHARMACY www.irjponline.com ISSN 2230 8407 Research Article STUDIES ON ANTIMICROBIAL ACTIVITY AND PHYTOCHEMICAL ANALYSIS OF CITRUS FRUIT JUICES AGAINST SELECTED ENTERIC
Chapter V SUMMARY AND CONCLUSION
 Chapter V SUMMARY AND CONCLUSION Coffea is economically the most important genus of the family Rubiaceae, producing the coffee of commerce. Coffee of commerce is obtained mainly from Coffea arabica and
Chapter V SUMMARY AND CONCLUSION Coffea is economically the most important genus of the family Rubiaceae, producing the coffee of commerce. Coffee of commerce is obtained mainly from Coffea arabica and
CHAPTER 1 INTRODUCTION
 CHAPTER 1 INTRODUCTION 1.1. Background Bread is one of the most widely-consumed food products in the world and breadmaking technology is probably one of the oldest technologies known. This technology has
CHAPTER 1 INTRODUCTION 1.1. Background Bread is one of the most widely-consumed food products in the world and breadmaking technology is probably one of the oldest technologies known. This technology has
Product Name and Number: 2372 Hot & Spicy Document #: Revision Date: 03/05/2015 Revision #: 1.0 Revision Reason: New Form Reviser: JM
 Product Overview: Dry, free flowing powder produced with a uniform savory hot spicy flavor. Salt based product able to maintain quality and consistency of color and flavor. Product Physical Properties
Product Overview: Dry, free flowing powder produced with a uniform savory hot spicy flavor. Salt based product able to maintain quality and consistency of color and flavor. Product Physical Properties
LACTIC ACID BACTERIA (OIV-Oeno , Oeno )
 LACTIC ACID BACTERIA (OIV-Oeno 328-2009, Oeno 494-2012) 1. OBJECT, ORIGIN AND FIELD OF APPLICATION Lactic acid bacteria are used in oenology to perform malolactic fermentation. The lactic acid bacteria
LACTIC ACID BACTERIA (OIV-Oeno 328-2009, Oeno 494-2012) 1. OBJECT, ORIGIN AND FIELD OF APPLICATION Lactic acid bacteria are used in oenology to perform malolactic fermentation. The lactic acid bacteria
How yeast strain selection can influence wine characteristics and flavors in Marquette, Frontenac, Frontenac gris, and La Crescent
 How yeast strain selection can influence wine characteristics and flavors in Marquette, Frontenac, Frontenac gris, and La Crescent Katie Cook, Enologist, University of Minnesota Fermentation Yeast Saccharomyces
How yeast strain selection can influence wine characteristics and flavors in Marquette, Frontenac, Frontenac gris, and La Crescent Katie Cook, Enologist, University of Minnesota Fermentation Yeast Saccharomyces
GROUP LA GARDONNENQUE. La Gardonnenque SCA since INOSUD SA since people. 25 M Turnover
 GROUP LA GARDONNENQUE La Gardonnenque SCA since 1969 INOSUD SA since 2000 90 people 25 M Turnover TRADITIONAL PRODUCTS OENOLOGY Alcohol Seeds Grape Seed Oil Calcium Tartrate Tartaric Acid Compost, Pulps,
GROUP LA GARDONNENQUE La Gardonnenque SCA since 1969 INOSUD SA since 2000 90 people 25 M Turnover TRADITIONAL PRODUCTS OENOLOGY Alcohol Seeds Grape Seed Oil Calcium Tartrate Tartaric Acid Compost, Pulps,
ENARTIS NEWS WANT TO PRODUCE A WINE WITH LOW OR ZERO SO 2
 ENARTIS NEWS WANT TO PRODUCE A WINE WITH LOW OR ZERO SO 2 ADDITION? SO 2 is one of the most controversial additives currently used in the wine industry. Numerous attempts have been made to find alternatives
ENARTIS NEWS WANT TO PRODUCE A WINE WITH LOW OR ZERO SO 2 ADDITION? SO 2 is one of the most controversial additives currently used in the wine industry. Numerous attempts have been made to find alternatives
Antibacterial Activity of Green Tea Leaves
 International Journal of Current Microbiology and Applied Sciences ISSN: 2319-7706 Volume 5 Number 11 (2016) pp. 472-477 Journal homepage: http://www.ijcmas.com Original Research Article http://dx.doi.org/10.20546/ijcmas.2016.511.054
International Journal of Current Microbiology and Applied Sciences ISSN: 2319-7706 Volume 5 Number 11 (2016) pp. 472-477 Journal homepage: http://www.ijcmas.com Original Research Article http://dx.doi.org/10.20546/ijcmas.2016.511.054
Post-Harvest-Multiple Choice Questions
 Post-Harvest-Multiple Choice Questions 1. Chilling injuries arising from the exposure of the products to a temperature a. above the normal physiological range b. below the normal physiological range c.under
Post-Harvest-Multiple Choice Questions 1. Chilling injuries arising from the exposure of the products to a temperature a. above the normal physiological range b. below the normal physiological range c.under
DOWNLOAD OR READ : YEAST STRESS RESPONSES 1ST EDITION PDF EBOOK EPUB MOBI
 DOWNLOAD OR READ : YEAST STRESS RESPONSES 1ST EDITION PDF EBOOK EPUB MOBI Page 1 Page 2 yeast stress responses 1st edition yeast stress responses 1st pdf yeast stress responses 1st edition Yeast Stress
DOWNLOAD OR READ : YEAST STRESS RESPONSES 1ST EDITION PDF EBOOK EPUB MOBI Page 1 Page 2 yeast stress responses 1st edition yeast stress responses 1st pdf yeast stress responses 1st edition Yeast Stress
Tofu is a high protein food made from soybeans that are usually sold as a block of
 Abstract Tofu is a high protein food made from soybeans that are usually sold as a block of wet cake. Tofu is the result of the process of coagulating proteins in soymilk with calcium or magnesium salt
Abstract Tofu is a high protein food made from soybeans that are usually sold as a block of wet cake. Tofu is the result of the process of coagulating proteins in soymilk with calcium or magnesium salt
10. THE ROLE OF PLANT GROWTH REGULATORS IN THE DEVELOPMENT, GROWTH AND MATURATION OF THE FRUIT
 The Division of Subtropical Agriculture. The Volcani Institute of Agricultural Research 1960-1969. Section B. Avocado. Pg 77-83. 10. THE ROLE OF PLANT GROWTH REGULATORS IN THE DEVELOPMENT, GROWTH AND MATURATION
The Division of Subtropical Agriculture. The Volcani Institute of Agricultural Research 1960-1969. Section B. Avocado. Pg 77-83. 10. THE ROLE OF PLANT GROWTH REGULATORS IN THE DEVELOPMENT, GROWTH AND MATURATION
Late-season disease control options to manage diseases, but minimize fermentation problems and wine defects
 Late-season disease control options to manage diseases, but minimize fermentation problems and wine defects Tony Wolf, Virginia Tech 1 Late-season disease control options to manage diseases..but minimize
Late-season disease control options to manage diseases, but minimize fermentation problems and wine defects Tony Wolf, Virginia Tech 1 Late-season disease control options to manage diseases..but minimize
Molecular identification of bacteria on grapes and in must from Small Carpathian wine-producing region (Slovakia)
 Molecular identification of bacteria on grapes and in must from Small Carpathian wine-producing region (Slovakia) T. Kuchta1, D. Pangallo2, Z. Godálová1, A. Puškárová2, M. Bučková2, K. Ženišová1, L. Kraková2
Molecular identification of bacteria on grapes and in must from Small Carpathian wine-producing region (Slovakia) T. Kuchta1, D. Pangallo2, Z. Godálová1, A. Puškárová2, M. Bučková2, K. Ženišová1, L. Kraková2
FOXP3 EXPRESSION IN HUMAN CANCER CELLS
 FOXP3 EXPRESSION IN HUMAN CANCER CELLS Vaios Karanikas EU Marie Curie Fellow Cancer Immunology Unit Department of Immunology and Histocompatibility School of Medicine University of Thessaly Larissa, Greece
FOXP3 EXPRESSION IN HUMAN CANCER CELLS Vaios Karanikas EU Marie Curie Fellow Cancer Immunology Unit Department of Immunology and Histocompatibility School of Medicine University of Thessaly Larissa, Greece
F&N 453 Project Written Report. TITLE: Effect of wheat germ substituted for 10%, 20%, and 30% of all purpose flour by
 F&N 453 Project Written Report Katharine Howe TITLE: Effect of wheat substituted for 10%, 20%, and 30% of all purpose flour by volume in a basic yellow cake. ABSTRACT Wheat is a component of wheat whole
F&N 453 Project Written Report Katharine Howe TITLE: Effect of wheat substituted for 10%, 20%, and 30% of all purpose flour by volume in a basic yellow cake. ABSTRACT Wheat is a component of wheat whole
ANTIMICROBIAL EFFECT OF SOUR POMEGRANATE SAUCE ON KISIR, A TRADITIONAL APPETIZER
 ANTIMICROBIAL EFFECT OF SOUR POMEGRANATE SAUCE ON KISIR, A TRADITIONAL APPETIZER Şeniz KARABIYIKLI 1, Duygu KIŞLA 2, Şahika E. A.GÖNÜL 2 1 Gaziosmanpaşa University, Faculty of Engineering and Natural Sciences,
ANTIMICROBIAL EFFECT OF SOUR POMEGRANATE SAUCE ON KISIR, A TRADITIONAL APPETIZER Şeniz KARABIYIKLI 1, Duygu KIŞLA 2, Şahika E. A.GÖNÜL 2 1 Gaziosmanpaşa University, Faculty of Engineering and Natural Sciences,
Unit code: A/601/1687 QCF level: 5 Credit value: 15
 Unit 24: Brewing Science Unit code: A/601/1687 QCF level: 5 Credit value: 15 Aim This unit will enable learners to apply knowledge of yeast physiology and microbiology to the biochemistry of malting, mashing
Unit 24: Brewing Science Unit code: A/601/1687 QCF level: 5 Credit value: 15 Aim This unit will enable learners to apply knowledge of yeast physiology and microbiology to the biochemistry of malting, mashing
Further investigations into the rind lesion problems experienced with the Pinkerton cultivar
 Further investigations into the rind lesion problems experienced with the Pinkerton cultivar FJ Kruger and SD Mhlophe Agricultural Research Council Institute for Tropical and Subtropical Crops Private
Further investigations into the rind lesion problems experienced with the Pinkerton cultivar FJ Kruger and SD Mhlophe Agricultural Research Council Institute for Tropical and Subtropical Crops Private
l?\ DEVELOPMENT OF CARBONATED HERBAL NELLI DRINK 1~~9647 Kushan Chanaka Amarasinghe p.,101)..'\
 ~-- ----------.-... p.,101)..'\ l?\ 0\' rj;) o DEVELOPMENT OF CARBONATED HERBAL NELLI DRINK (Phyllanthus emblica.) By Kushan Chanaka Amarasinghe Llbary - USJP 1111I11111 111111 199647 B.Sc. (Sp.) in Food
~-- ----------.-... p.,101)..'\ l?\ 0\' rj;) o DEVELOPMENT OF CARBONATED HERBAL NELLI DRINK (Phyllanthus emblica.) By Kushan Chanaka Amarasinghe Llbary - USJP 1111I11111 111111 199647 B.Sc. (Sp.) in Food
How to fine-tune your wine
 How to fine-tune your wine Fining agents help remove undesirable elements or compounds to improve the quality of wine. Fining is not just used in wines for bottle preparation, in some cases there are more
How to fine-tune your wine Fining agents help remove undesirable elements or compounds to improve the quality of wine. Fining is not just used in wines for bottle preparation, in some cases there are more
Prickly Pear Research Information
 Prickly Pear Research Information THIS INFORMATION DOES NOT CONSTITUTE ADVERTISING FOR ANY PRODUCT. This information is intended for physicians and other licensed health care provider to use as a basis
Prickly Pear Research Information THIS INFORMATION DOES NOT CONSTITUTE ADVERTISING FOR ANY PRODUCT. This information is intended for physicians and other licensed health care provider to use as a basis
COMPARATIVE STUDY OF ANTIOXIDANT POTENTIAL OF TEA WITH AND WITHOUT ADDITIVES
 Indian J Physiol Pharmacol 2000; 44 (2): 215-219 COMPARATIVE STUDY OF ANTIOXIDANT POTENTIAL OF TEA WITH AND WITHOUT ADDITIVES SUNITA TEWARI,* VAN I GUPTA AND SANDEEP BHATTACHARYA Dep artmen.t of Physiology,
Indian J Physiol Pharmacol 2000; 44 (2): 215-219 COMPARATIVE STUDY OF ANTIOXIDANT POTENTIAL OF TEA WITH AND WITHOUT ADDITIVES SUNITA TEWARI,* VAN I GUPTA AND SANDEEP BHATTACHARYA Dep artmen.t of Physiology,
GAS-CHROMATOGRAPHIC ANALYSIS OF SOME VOLATILE CONGENERS IN DIFFERENT TYPES OF STRONG ALCOHOLIC FRUIT SPIRITS
 GAS-CHROMATOGRAPHIC ANALYSIS OF SOME VOLATILE CONGENERS IN DIFFERENT TYPES OF STRONG ALCOHOLIC FRUIT SPIRITS Vesna Kostik 1*, Shaban Memeti 1, Biljana Bauer 2 1* Institute of Public Health of Republic
GAS-CHROMATOGRAPHIC ANALYSIS OF SOME VOLATILE CONGENERS IN DIFFERENT TYPES OF STRONG ALCOHOLIC FRUIT SPIRITS Vesna Kostik 1*, Shaban Memeti 1, Biljana Bauer 2 1* Institute of Public Health of Republic
Wine and Health. Mickey Parish, Ph.D. Professor and Chair Dept of Nutrition and Food Science College of Agriculture and Natural Resources
 Wine and Health Mickey Parish, Ph.D. Professor and Chair Dept of Nutrition and Food Science College of Agriculture and Natural Resources "Nothing more excellent or valuable than wine was ever granted by
Wine and Health Mickey Parish, Ph.D. Professor and Chair Dept of Nutrition and Food Science College of Agriculture and Natural Resources "Nothing more excellent or valuable than wine was ever granted by
Development of compost tea production method
 Development of compost tea production method Compost Council of Canada, Niagara Falls, Ontario Yves Bernard, eng., project manager September 26-28 2016 Presentation outline CRIQ Background Methodology
Development of compost tea production method Compost Council of Canada, Niagara Falls, Ontario Yves Bernard, eng., project manager September 26-28 2016 Presentation outline CRIQ Background Methodology
Timing of Treatment O 2 Dosage Typical Duration During Fermentation mg/l Total Daily. Between AF - MLF 1 3 mg/l/day 4 10 Days
 Micro-Oxygenation Principles Micro-oxygenation is a technique that involves the addition of controlled amounts of oxygen into wines. The goal is to simulate the effects of barrel-ageing in a controlled
Micro-Oxygenation Principles Micro-oxygenation is a technique that involves the addition of controlled amounts of oxygen into wines. The goal is to simulate the effects of barrel-ageing in a controlled
Effects of Acai Berry on Oatmeal Cookies
 Jessica Dooley and Jennifer Gotsch FN 453 Team Project Written Report Effects of Acai Berry on Oatmeal Cookies Abstract: Oxidative stress can cause many diseases such as cancer, heart disease, and stoke.
Jessica Dooley and Jennifer Gotsch FN 453 Team Project Written Report Effects of Acai Berry on Oatmeal Cookies Abstract: Oxidative stress can cause many diseases such as cancer, heart disease, and stoke.
Red Wine and Cardiovascular Disease. Does consuming red wine prevent cardiovascular disease?
 Red Wine and Cardiovascular Disease 1 Lindsay Wexler 5/2/09 NFSC 345 Red Wine and Cardiovascular Disease Does consuming red wine prevent cardiovascular disease? Side 1: Red wine consumption prevents cardiovascular
Red Wine and Cardiovascular Disease 1 Lindsay Wexler 5/2/09 NFSC 345 Red Wine and Cardiovascular Disease Does consuming red wine prevent cardiovascular disease? Side 1: Red wine consumption prevents cardiovascular
Effectiveness of the CleanLight UVC irradiation method against pectolytic Erwinia spp.
 Page 1 of 12 Effectiveness of the CleanLight UVC irradiation method against pectolytic Erwinia spp. Zon Fruit & Vegetables Author: Agnieszka Kaluza Innovation & Development Engineer 29 November 2013 Versie:
Page 1 of 12 Effectiveness of the CleanLight UVC irradiation method against pectolytic Erwinia spp. Zon Fruit & Vegetables Author: Agnieszka Kaluza Innovation & Development Engineer 29 November 2013 Versie:
Forestry, Leduc, AB, T9E 7C5, Canada. Agriculture/Forestry Centre, Edmonton, AB T6G 2P5, Canada. *
 Effect of High Pressure Processing on Quality, Sensory Acceptability and Microbial Stability of Marinated Beef Steaks and Pork Chops during Refrigerated Storage Haihong Wang 1 *, Jimmy Yao 1 Mindy Gerlat
Effect of High Pressure Processing on Quality, Sensory Acceptability and Microbial Stability of Marinated Beef Steaks and Pork Chops during Refrigerated Storage Haihong Wang 1 *, Jimmy Yao 1 Mindy Gerlat
INDIAN COUNCIL OF AGRICULTURAL RESEARCH DIRECTORATE OF RAPESEED-MUSTARD RESEARCH, BHARATPUR, INDIA
 INDIAN COUNCIL OF AGRICULTURAL RESEARCH DIRECTORATE OF RAPESEED-MUSTARD RESEARCH, BHARATPUR, INDIA Pathogenic variability of Sclerotinia sclerotiorum isolates on Brassica differentials Pankaj Sharma ICAR-Directorate
INDIAN COUNCIL OF AGRICULTURAL RESEARCH DIRECTORATE OF RAPESEED-MUSTARD RESEARCH, BHARATPUR, INDIA Pathogenic variability of Sclerotinia sclerotiorum isolates on Brassica differentials Pankaj Sharma ICAR-Directorate
